Chemistry Nanomaterials and Bonding Test - Yr 11 ATAR 2025
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
60 Terms
What are nanomaterials?
. substances that contain particles in the size range 1-100nm + have specific properties relating to the size of these particles which may differ from those of the bulk material
Where are nanomaterials found in?
. cosmetics, medications, food, electronics
What are the 2 key features of nanomaterials?
Extremely small: can be small enough to pass through cell membrane
Large SA to volume ratio: compared to the bulk material
—> speeds up the rate of a reaction + increases their effectiveness as catalysts as reactions take place on the surface of materials
What is the difference of nanomaterials to their bulk material?
. nanomaterials may differ in terms of their physical + chemical properties, such as colour, electrical conductivity, reactivity and melting point
—> bulk gold is non reactive, whereas nanogold is highly reactive, the colour of nanogold varies based on the structure of the nanoparticle
Explain why and how nanomaterials are regulated in products
. are relatively new, meaning we have limited data regarding long term effects on human health, safety, and the environment
—> their use is thus regulated by various bodies in Aus: food standards Australia New Zealand (FSANZ) regulates nanotechnologies in foods, food packaging, and food contact materials
Discuss the application of nanomaterials in cosmetic products
. Zinc oxide + titanium dioxide nanoparticles can make sunscreen more effective + appear invisible instead of white
—> HOWEVER: scientists are unsure if the nanomaterials can enter our bodies through our skin, and whether they’re damaging the environment (eg, getting into our waterways, washing off in the ocean)
Outline the application of 2 Carbon nanomaterials
Buckminsterfullerene (bucky ball): medical applications such as antiviral agent + drug delivery
Carbon nanotubes: exceptionally strong + conductive, used in electronics
Bonding and the Periodic Table General 3 facts
Atoms tend to form chemical bonds to obtain a filled valence shell + become chemically stable
The no. of electrons in valence shells can be determined from periodic table
Elements in same group have same no. of valence electrons
How do chemical bonds form?
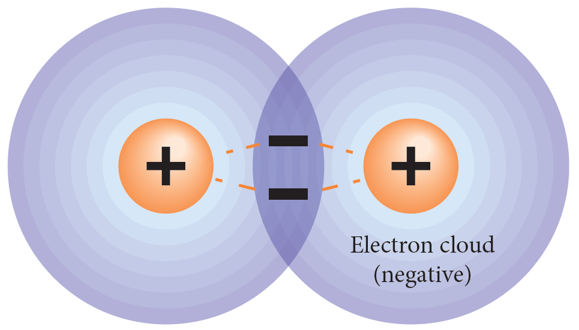
. chemical bonds form due to the electrostatic force of attraction between positive and negative charges in participating atoms
—> the electrons in different atoms experience a force of attraction from both nuclei
—> the negative-positive-negative attraction holds the 2 particles together
—> this attraction = a chemical bond
—> 1 pair of electrons = 1 bond
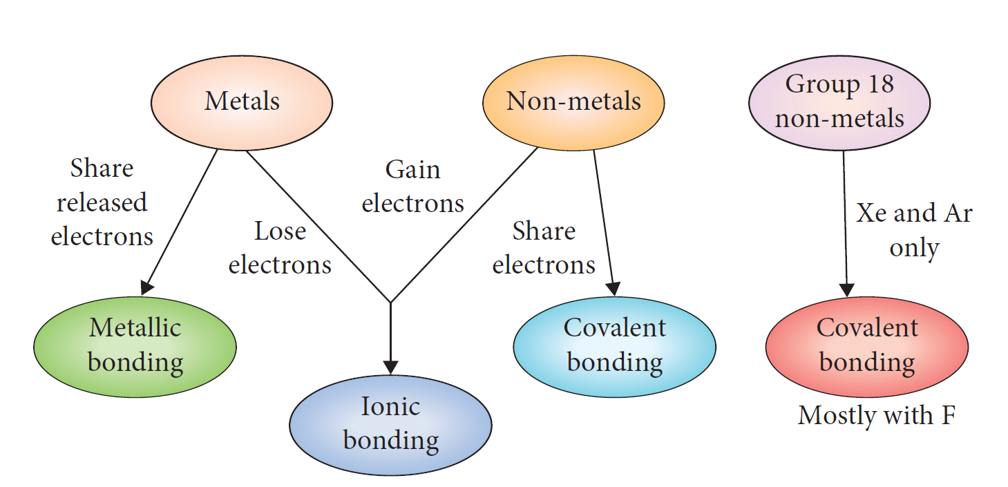
3 types of bonds
What are 4 metallic character trends on the periodic table?
Metals make up 75% of elements on periodic table
Metals lose valence electrons to form positive ions
Elements toward the left of table have increasing metallic character
—> as nucleus of elements on left have a smaller positive charge than elements on right, resulting in lower force on their valence electrons (thus easier to lose)
Metallic character increases moving down the group
—> as electron shielding causes the atomic radius to increase, thus the outer electrons ionise more readily than electrons in smaller atoms
Describe Metallic bonding
. the electrostatic forces of attraction between the metal cations, arranged in a lattice structure, and the delocalised electrons around them
—> when bonded together, the valence electrons of metallic atoms delocalise
—> leaves metal cations surrounded by a sea of free roaming electrons that are free to move within the regular lattice structure
—> the bonding is NON-directional
—> can be modelled as a regular arrangement of atoms with electrostatic forces of attraction between the nuclei of these atoms and their delocalised electrons that are able to move within the 3D lattice
Metallic Properties: 1. Metallic Lustre
. Mirror-like shininess
—> light rays reflect off the delocalised electrons + close packing of the metal cations prevent light from passing through, making the metal opaque
Metallic Properties: 2. Good conductor of heat
. allows heat to travel from one end to the other
—> the mobile electrons acquire energy from the heat source + move with far greater speed
—> they collide with other electrons transferring energy
—> ions will vibrate vigorously, pushing their neighbours to vibrate as well, rapidly transferring the heat energy
Metallic Properties: 3. Good conductor of electricity
. allows an electric current to easily pass through
—> an electric current is the overall movement of charged particles in 1 direction
—> the highly mobile delocalised electrons within the lattice are free to move
—> when attached to a power supply the electrons will move to the positive terminal
Metallic Properties: 4. Malleable and Ductile
. Malleable: able to be beaten into another shape or flattened into a thin sheet with a hammer without breaking
. Ductile: able to be drawn out into a wire
—> metal cations can move past each other if enough force is applied
—> electrostatic attraction between the metal cations and delocalised electrons is non directional
—> thus, the substance can change shape without disrupting the bonding
Metallic Properties: 5. High M.P and B.P
. as metals are giant lattice structures, the no. of electrostatic forces to be broken is extremely large = high M.P + B.P
—> means that the M.P and B.P of metals are more similar to those for ionic compounds than for covalent substances
What is a metal alloy?
. a substance that combines more than 1 metal or mixes a metal with other non-metallic elements
—> typically homogenous mixtures
Metal alloy: 1. Steel
. 95%+ iron and small amount of carbon
—> stronger than iron and used in construction + manufacturing
Metal alloy: 2. Bronze
. copper with 12-12.5% tin
—> used in history for weapon production but soon replaced with iron
—> modern uses: small parts + architecture
Metal Alloy: 3. Alloyed gold
. varying percentages of gold with other metals to improve durability of jewellery
What are ions, how do they form, and what ions do metals and non-metals form?
Ions are atoms or groups of atoms that are electrically charged due to an imbalance in the number of electrons + protons
this imbalance can occur due to atoms donating (losing) and receiving (gaining) electrons to attain a full valence shell
Metals donate their valence electrons, forming positive ions (cations), whereas non-metals receive ions, forming negative ions (anions)
Describe ionic bonding
. a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions formed when electrons are transferred from one atom to another
—> occurs between a metal and a non-metal
—> metal donates valence electrons and non-metal receives them
—> this process results in an orderly array of positive + negative ions = a lattice
—> lattice is very strong due to the electrostatic force of attraction between oppositely charged ions
Briefly describe the steps necessary for an ionic bond to form
Formation of ions
attraction between opposite charges
formation of an ionic compound
Q: Describe in terms of electron behaviour, how ionic bonds form between the elements Magnesium and Sulfur
Mg atoms lose 2 electrons
Sulfur atoms gain 2 electrons
Mg2+ cations and S2- anions are formed
Oppositely charged ions are electrostatically attracted to one another (forming ionic bonds)
What are the properties of ionic substances due to in general
. due to the lattice of charged ions
Properties of Ionic compounds: 1. High M.P and B.P
. there’s strong attraction between ions, so a lot of energy is needed to move the ions out of their fixed position in the lattice
—> a lot of energy is required to overcome the attraction between the positive and negative ions in ionic compounds
—> thus, high temp (energy) required to melt ionic compounds or cause them to boil
—> ions with a greater charge will allow for higher electrostatic attraction with other ions in lattice, thus the more electrons donated or received = stronger the charge
. APPLICATION: Production of substances such as glass
Properties of Ionic compounds: 2. Conducts electricity and heat when in Molten and Aqueous state
. charged particles are free to move in the molten + aqueous state but are held in a fixed position in the solid states
—> conducting electricity requires mobile charged particles
—> when ionic compounds are dissolved in water the dissociated ions are free to conduct electric charge through the solution
—> molten ionic compounds (molten salts) also conduct electricity
—> thus ionic substances are good insulators when solid, but not when aqueous/liquid state
APPLICATION: Batteries
Properties of Ionic compounds: 3. Hard and Brittle
. ions are held rigidly in place in the lattice but the application of stress brings ions of the same charge close together so the crystal can shatter
—> ionic crystals hard as the positive and negative ions are strongly attracted to each other + difficult to separate
—> when pressure applied to an ionic crystal then ions of like charge may be forced closer to each other
—> the electrostatic repulsion can be enough to split the crystal = brittle
APPLICATION: Abrasives
Properties of Ionic compounds: 4. Mostly soluble in water
. ions are attracted to the water molecules + move out of position
—> ionic compounds dissociate and dissolve in polar solvents
—> the positive cation from the ionic solid is attracted to the negative end of the water molecule (oxygen) and the negative anion of the ionic solid is attracted to the positive end of the water molecule (hydrogen)
Properties of Ionic compounds: 5. Giant Crystal lattice of repeating ions
. electrostatic attraction between positive and negative charges causes ions of opposite charge to surround each other
—> ionic compounds form crystal lattices rather than amorphous solids
—> at an atomic level, an ionic crystal is a regular structure, with the cation and anion alternating with each other + forming a 3D structure based largely on the smaller ion evenly filling in the gaps between the larger ion
How do ratios relate to ionic substances, and how are they useful?
. ionic substances have fixed ratios based on how many of each element is required for all ions to have a full valence shell
—> NaCl= 1 sodium for every 1 chlorine
—> MgCl2= 1 Mg for every 2 Cl
—> Useful: it lets you know how much of each reactant you will need to produce a product, as well as calculating percentage composition
Describe covalent bonding
. a DIRECTIONAL chemical bond that involves the sharing of electrons between 2 atoms to form electron pairs between the atoms
—> non-metal atoms have high electronegativity
—> covalent bond occurs whenever non-metal elements bond to other non-metal elements
—> atoms share electrons so that each atom involved may form a stable octet or electron configuration
—> they’re then held together by electrostatic attraction between the shared electrons and the nuclei of adjacent atoms
—> directional = the shared electrons are located between the 2 atoms, creating a specific alignment or direction
Why do atoms share valence electrons in covalent bonding?
. in order to get a stable full octet (or duet for H)
—> every non metal element wants 8 valence electrons (except for H which only wants 2)
Compare the 2 different types of substances which contain covalent bonds
Covalent Network substances:
—> contain an infinite number of atoms joined by covalent bonds in a giant network
Covalent Molecular substances:
—> contain discrete molecules which consist of a number of atoms joined by covalent bonds
—> they’re non-metal elements or compounds of non-metals
—> H2O, CO2, N2, O2, NH3
Outline the features of a covalent molecular substance
. the forces joining atoms together in a molecule (intramolecular forces) are covalent bonds
—> these are very strong
. the forces between molecules = intermolecular forces
—> are very weak
Properties of Covalent Molecular Substances: 1. Low (to moderate) M.P and B.P
. are often liquids or gases at room temp
—> the weak intermolecular forces between molecules can be overcome easily
. when a molecular substance melts or boils, only the weak intermolecular forces (forces between molecules) need to be broken or overcome
—> thus, the weakly bonded lattice of molecules in the solid phase is easily disrupted by heat energy to form a liquid or gas
*The strong covalent bonds occurring between the atoms within the molecules (intramolecular forces) are unaffected when a substance melts or boils
Properties of Covalent Molecular Substances: 2. Non-conductors of electricity
. absence of mobile charges to carry the current
. electrons are localised within the bond and the substance doesn’t contain ions
. electrons are localised within each atom’s electron cloud or as shared electrons within covalent bonds
—> none of these electrons are free to move independently
—> substances DO NOT contain ions
—> the absence of any freely mobile charged particles = non conductor of electricity
Properties of Covalent Molecular Substances: 3. Form solids which are soft + weak with a waxy appearance
. due to weak intermolecular forces
. strong covalent bonds (intramolecular forces) only form between the atoms within molecules
—> only weak intermolecular forces of attraction occur between neighbouring molecules
—> thus, molecules are easily separated from one another = substances are weak + soft
Explain the electron dot diagrams
. atoms involved in covalent bonds gain a noble gas configuration (8 valence electrons) hence the octet rule
—> this rule predicts that atoms form enough covalent bonds to surround themselves with 8 electrons each
—> electrons pairs involved in bonds = bonding pairs, those not involved = non-bonding pairs
Describe the different multiple covalent bonds?
. a covalent bond in which there are 2 pairs of electrons shared = double covalent bond
—> allows both the atoms to achieve a stable electron configuration, eg CO2
. a covalent bond in which there are 3 pairs of electrons shared = a triple covalent bond, eg nitrogen gas
What are coordinate covalent bonds?
. A coordinate bond is a covalent bond (a shared pair of electrons) in which both electrons come from the same atom
—> sometimes they occur when elements in groups 15,16 and 17 retain non bonding pairs of electrons
—> these electrons may be shared with other atoms which have vacant valence shells to form additional covalent bonds
EG: sulfur dioxide
Provide examples of applications of covalent compounds
*UNDONE
. Butter = soft so we can spread it easily
. Pharmaceuticals: many medications, including antidepressants and pain relievers, are covalent compounds, and their solubility properties are important for drug delivery.
Describe Covalent Network Bonding
. A chemical compound (or element) in which the atoms are bonded by covalent bonds in a continuous network extending throughout the material
—> atoms in these substances form covalent bonds with multiple neighbouring atoms resulting in a continuous array of covalently bonded atoms
Difference between covalent network structure and covalent molecular structure
. In network, the strong covalent bonding extends continuously throughout the substance, while in molecular, the strong covalent bonds only occur between the few atoms within a molecule while weak intermolecular forces occur between molecules
. Covalent network structures form vast, continuous 3D networks of atoms held together by strong covalent bonds, leading to high melting points and hardness - EG Diamond
. Covalent molecular structures consist of discrete molecules held together by weaker intermolecular forces, resulting in lower melting points and softer materials - EG Water
Describe an example of the covalent network structure
. carbon + silicon have 4 valence electrons so they can form 4 covalent bonds
—> they exist as 3D networks of covalently bonded atoms where each atom is covalently bonded to 4 other atoms
—> covalent network substances contain NO molecules
Properties of Covalent Network Substances: 1. Very High M.P + B.P
. covalent bonds are very difficult to break
. strong covalent bonds occur between all atoms within the structure = a very high temp (high particle kinetic energy) is needed to disrupt this continuous array of strongly bonded atoms
Properties of Covalent Network Substances: 2. Non-conductors of electricity (or heat)
. no mobile charged particles to carry the current
. electrons are held in fixed positions within the atom’s shells, lone pairs or covalent bonds
—> as the electrons aren’t free to move independently they are unable to conduct electricity or heat through the substance
. Exception = Graphite, contains some delocalised valence electrons that can move freely throughout the structure = good conductor of electricity
Properties of Covalent Network Substances: 3. Very Hard and Brittle
. strong directional bonds prevent atoms from moving, so when stress is applied, the bonds break rather than allowing the structure to deform
. strong covalent bonds occur between all atoms
—> this continuous array of strongly bonded atoms is difficult to disrupt = hard + brittle
. Exception: Graphite - atoms are covalently bonded into rings of 6 carbon atoms, these rings interlock to form strongly bonded, flat, 2D layers
—> as only weak bonding forces occur between these layers they are able to slip over one another with ease = soft material
Properties of Covalent Network Substances: 4. Chemically inert (unreactive)
. no free electrons to participate in reactions
Properties of Covalent Network Substances: 5. Insoluble
. covalent bonds are difficult to break so substances don’t ionise
Describe the features of Silicon
. a grey, brittle semi-metallic solid
. same valence electron configuration as C and exists in a structure similar to diamond
. Uses: solar cells + the electronics industry
Describe the features of Silicon Dioxide (Quartz/Silica)
. the most abundant compound on Earth, eg S
. SiO2 has each Si atom bonded to 4 O atoms in a tetrahedral arrangement + each O atom bonded to 2 Si atoms
. hard with a high M.P as the bonding extends through the entire lattice
. USES: quartz watches + crystal microphones
What are allotropes?
. different forms of the same element which exhibit different physical properties
Describe diamond as an allotrope of carbon
. a crystalline allotrope of carbon, and each crystal can be pictured as a single giant molecule made up of a regular network of carbon atoms
Describe the bonding within diamond and how this results in its properties
. there are 4 covalent bonds at each atom
—> these tetrahedral bonds exist throughout the crystal + give it a rigid structure
. the bonding electrons are tightly bound + highly localised, so electricity is NOT conducted
. Difficult to distort/shatter: involves breaking many covalent bonds, so diamond has a high M.P + B.P and is the hardest substance known
4 Uses of diamond
Gemstones
glass cutting and polishing
dentist’s drills
Record player needles
Describe Graphite as another allotrope of carbon and its properties
. a 2nd allotrope of C
. has a high M.P + B.P
—> BUT is an electrical conductor + is quite soft due to the free electrons
Describe the bonding present in Graphite
. each C atom is bonded to 3 other C atoms in a hexagonal structure to form a flat 2D layer
—> these layers are stacked on top of each other
. The 4th electron from each C atom is delocalised + can move within the layers allowing conduction of electricity
. the bonding between layers is weak so they can slide easily over one another
What are some applications of Graphite
Lead pencils
sporting equipment (tennis rackets, golf clubs)