Chapter 23: Polymers and Alcohols
23.1-Addition Polymers
Polymers
- Polymers are long molecules made up of hundreds or thousands of repeating subunits.
- They are really useful materials for everyday items, such as plastic containers and clothing, but they are a nuisance when it comes to throwing them away because they aren’t broken down easily.
Addition Polymerisation
- Addition polymers are formed by joining lots of alkene molecules together.
- The single alkene is called a monomer and when several monomers are connected together, we call this a polymer.
- Polymerisation happens when the double carbon bond breaks, allowing another alkene to connect to the carbon.
- This happens multiple times until you end up with a polymer made up of hundreds or thousands of monomers.
- Instead of drawing out a really long chain, it’s much easier to represent polymers by drawing a single subunit (the monomer) inside square brackets, with a little ‘n’ in the right hand corner to show that we have lots of them joined together.
- Remember to draw bonds sticking outside the square brackets to make it clear that the chain continues.
- We name the polymer depending on the type of monomer it is made from and stick the word poly at the front.
- Let’s say we have a polymer made up of lots of ethene molecules joined together - this would be called poly(ethene), which we also refer to as polythene.
- Polythene is everywhere - you’ll find it in things like plastic water bottles, bin liners and hose pipes.
23.2-Alcohols
Alcohols
- Functional Groups
- The ‘O-H’ bond is the functional group of alcohols. Alcohols contain a bond between O-H, where the oxygen is bonded to a carbon. This is their functional group, used in reactions.
- As we have seen with alkanes and alkenes, alcohols can also be named. The first four alcohols in the homologous series are called methanol, ethanol, propanol and butanol.
Representing Alcohols
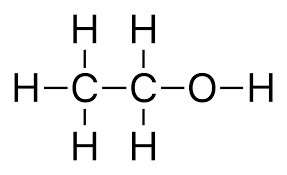
Alcohols can be represented. We can represent alkenes very easily, by using the number of carbons each molecule contains. Ethanol can be represented as CH3CH2OH.
Alcohols can be drawn out. We can also represent alcohols using their displayed formulae. For example, the displayed formulae of ethanol is shown below.
Production of Ethanol by Fermentation
- Ethanol (C2H5OH) is one of the most important alcohols
- It is the type of alcohol found in alcoholic drinks such as wine and beer
- It is also used as fuel for cars and as a solvent
- It can be produced by fermentation where sugar or starch is dissolved in water and yeast is added
- The mixture is then fermented between 15 and 35°C with the absence of oxygen for a few days
- Yeast contains enzymes that break down sugar to glucose
- If the temperature is too low the reaction rate will be too slow and if it is too high the enzymes will become denatured
- The yeast respire anaerobically using the glucose to form ethanol and carbon dioxide:
Practice Questions:
- Give an example of an alkene polymer.
- Polythene/propene/butene
- How are polymers made?
- Monomers join together by polymerisation
- Define condensation polymerisation.
- Condensation polymerisation involves monomers with two functional groups
- When these monomers react they join together usually lo sing small molecules, so are called condensation polymerisation reactions