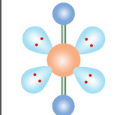
VSEPR Theory
1/40
Earn XP
Description and Tags
Naming the configurations, angles, and recognizing their shapes.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
41 Terms
Linear
If a molecule has 2 electron groups, what is it’s molecular configuration?
180
What is the degree angle between the electron groups in a linear molecule?
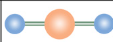
Linear
What is the name of this molecule’s configuration?
Trigonal Planar
If a molecule had 3 electron groups and no lone pairs, what is it’s molecular configuration?
120
What is the degree angle between electron groups of a trigonal planar molecule?
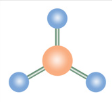
Trigonal Planar
What is the name of this molecular configuration?
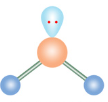
Bent
If a molecule has 3 electron groups and 1 is a lone pair, what is it’s molecular configuration?
>120
What is the degree angle between electron groups in a bent molecule (trigonal)?
Lone pairs push more on the bond, creating more space
Why is the degree angle between a lone pair and a bond greater than the distance between two bonds?
Tetrahedral
If a molecule has four electron pairs and no lone pairs, what is it’s molecular configuration?
109.5
What is the degree angle between electron groups in a tetrahedral molecule?
Trigonal Bent
What is the name of this molecular configuration?
Tetrahedral
What is the name of this molecular configuration?
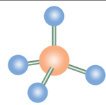
Trigonal Pyramidal
If a molecule has four electron groups and 1 is a lone pair, what is it’s molecular configuration?
107
What is the degree angle between electron groups in a trigonal pyramidal molecule?
Trigonal Pyramidal
What is the name of this molecular configuration?
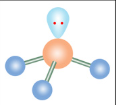
Bent
If a molecule has four electron groups and 2 are lone pairs, what is it’s molecular configuration?
104.5
What is the degree angle between electron groups in a bent molecule (tetrahedral)?
Bent
What is the name of this molecular configuration?
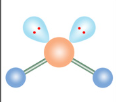
Trigonal Bipyramidal
If a molecule has five electron groups and no lone pairs, what is it’s molecular configuration?
90 and 120
What is the degree angle between electron groups in a trigonal bipyramidal molecule? (2)
Trigonal Bipyramidal
What is the name of this molecular configuration?
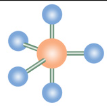
Sawhorse
If a molecule has five electron groups and 1 is a lone pair, what is it’s molecular configuration?
>90
What is the degree angle between electron groups in a sawhorse molecule?
Sawhorse
What is the name of this molecular configuration?
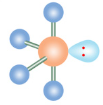
T-Shaped
If a molecule has five electron groups and two are lone pairs, what is it’s molecular configuration?
T-Shaped
What is the name of this molecular configuration?
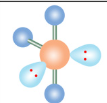
Linear
If a molecule has five electron groups and three are lone pairs, what is it’s molecular configuration?
Linear
What is the name of this molecular configuration?
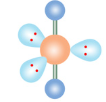
Octahedral
If a molecule has six electron groups and none are lone pairs, what is it’s molecular configuration?
90
What is the degree angle between electron groups in an octahedral molecule?
Octahedral
What is the name of this molecular configuration?
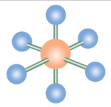
Square Pyramidal
If a molecule has six electron groups and one is a lone pair, what is it’s molecular configuration?
>90
What is the degree angle between electron groups in a square pyramidal molecule?
Square Pyramidal
What is the name of this molecular configuration?
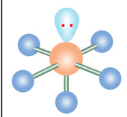
Square Planar
If a molecule has six electron groups and two are lone pairs, what is it’s molecular configuration?
Square Planar
What is the name of this molecular configuration?
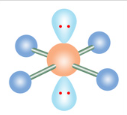
T-Shaped
If a molecule has six electron groups and three are lone pairs, what is it’s molecular configuration?
T-Shaped
What is the name of this molecular configuration?
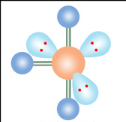
Linear
If a molecule has six electron groups and four are lone pairs, what is it’s molecular configuration?
Linear
What is the name of this molecular configuration?