Introduction to Antimicrobials (AKA Antibiotics)- Flashcards
1/126
Earn XP
Description and Tags
Flashcards
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
127 Terms
What is the main difference between Gram-positive and Gram-negative cell walls?
Gram-positive: Thick peptidoglycan + teichoic acids 🧱
Gram-negative: Thin peptidoglycan + outer membrane with LPS & porins 🚪
Which antibiotics target the bacterial cell wall (external boundaries)?
🟠 Beta-lactams (penicillins, cephalosporins, carbapenems)
🟢 Glycopeptides (vancomycin)
Which antibiotics target nucleic acids (DNA/RNA)?
📖 Fluoroquinolones → DNA gyrase/topoisomerase
Which antibiotics target the bacterial ribosomes?
50S inhibitors: Macrolides
30S inhibitors: Aminoglycosides, Tetracyclines,
What are the Main 4 cell wall inhibitors?
Penicillins, Cephalosporins, Carbapenems
Vancomycin
What is the mechanism of action of penicillins?
Bind to PBPs (penicillin-binding proteins) 🔑
Block transpeptidation → no cross-linking of peptidoglycan 🧱
Result: bacteria die (bactericidal) ☠
Why are penicillins considered bactericidal?
Because they destroy the cell wall → bacteria lyse 💥
Give examples of common penicillins.
Penicillin, Nafcillin, Ampicillin, Piperacillin 💊
How many different types of PCNs are there?
Natural PCNS
Anti-staphylococcal penicillin
Aminopenicillins
Antipseudomonal
What infections do natural PCNs(Pen G IM,IV or Pen VK (PO) treat?
Should they be used for Staph aureus?
🌀 Syphilis (Treponema pallidum)
🍗 Gas gangrene (Clostridium perfringens)
🤧 Strep infections (Group A, S. pneumoniae)
🐶 Pasteurella (animal bites)
💉 Neisseria species
❌ No → high resistance
🦠 Anti-Staphylococcal Penicillins
Q: Give Examples?
Q: What do they cover?
Q: Do they cover MRSA?
Nafcillin (IV), Dicloxacillin (PO), Oxacillin (PO), Methicillin (historical)
MSSA (Methicillin-sensitive Staph aureus)
Coagulase-negative Staph (CoNS)
Some Strep species
❌ No
🌊 Aminopenicillins (Ampicillin, Amoxicillin)
Q: Examples?
Q: What can be added to protect them from beta-lactamases to inhibit their action?
Q: What do they cover?
Q: What’s a classic first-line use?
Ampicillin (IV), Amoxicillin (PO)
Beta-lactamases inhibitors: Amoxicillin + Clavulanic acid = Augmentin; Ampicillin + Sulbactam = Unasyn
Gram-negatives: E. coli, H. influenzae, Moraxella catarrhalis, Gut anaerobes, Some Gram-positives
Ear infections (AOM), sinusitis, respiratory tract infections
👾 Anti-Pseudomonal Penicillin
Q: Example?
Q: What is it usually combined with and why? *think beta-lactamase
Q: Spectrum? Q: What do they cover?
Piperacillin (IV)
Given with beta-lactamase inhibitor. Tazobactam → Piperacillin-Tazobactam (Zosyn).
Broadest penicillin:
Gram-positives
Enteric Gram-negatives
Gut anaerobes
Pseudomonas aeruginosa
Which ENT infections are treated with Amoxicillin or Amoxicillin-Clavulanate (*Aminopenicillins )?
Acute Otitis Media (AOM) 👂
Sinusitis 🤧
Pharyngitis (Strep throat) 🗣
Common pathogens: Strep pneumo, H. flu, Moraxella
What is the 1st-line drug for sinusitis and AOM in children?
A: Amoxicillin (or Amoxicillin-Clavulanate) 🧒💊
What are the main adverse effects of penicillins?
Hypersensitivity reactions 🤧 (rash, anaphylaxis)
Jarisch–Herxheimer reaction 💥 (seen in syphilis treatment)
What causes the Jarisch–Herxheimer reaction?
A: Rapid lysis of spirochetes (Treponema pallidum) after penicillin treatment 🌀
What are the main contraindications to penicillin use?
Penicillin allergy 🚫
Severe renal impairment (especially with methicillin) 🚱
What class of drugs are cephalosporins in?
Beta-lactams (cell wall synthesis inhibitors) 🧱
Give examples of Beta-Lactams Cephalosporins
(*hint: think 5C’s)
Cefazolin (1st), Cefpodoxime, Ceftriaxone, Cefepime, Ceftazidime
Name two examples of 1st generation cephalosporins.
Do they have good coverage for GP bacteria? (MSSA, some CoNS species, Strep species).
Do 1st gens Cephalosporins have strong Gram-negative coverage?
Cefazolin (IV), Cephalexin (PO);
Yes; GP
No → limited Gram-negative coverage
Which Gram-positive organisms are targeted by 1st gen Cephalosporins such as, cefazolin (IV) and cephalexin (PO)?
MSSA, some CoNS species, Strep species
Coagulase-Negative Staphylococcus (CoNS)
Methicillin-Susceptible Staphylococcus Aureus (MSSA,)
Why should you avoid using 1st gens Cephalosporins empirically (guess) for staph infections?
High resistance rates
Empiric = Educated guess 🎯
Targeted = Lab-confirmed 🔬
💡 Example:
A child comes in with an ear infection 👂.
The doctor doesn’t yet know if it’s Strep pneumo, H. flu, or Moraxella.
So they empirically prescribe Amoxicillin — because it usually works for the most common causes.
Later, if a culture result shows it’s actually resistant H. flu, the doctor may switch to Amoxicillin-Clavulanate (targeted therapy).
Name four examples of 2nd generation cephalosporins.
Cefuroxime (PO/IV), Cefaclor (PO), also
Cefotetan and Cefoxitin (IV)- cover anaerobes
What extra coverage do 2nd gen cephalosporins (Beta-Lactams) have compared to 1st gen cephalosporins?
Better Gram-negative coverage
Which Gram-negative organisms are covered by 2nd gens?
H. influenzae, Klebsiella, Moraxella
Do 2nd gen cephalosporins keep the same Gram-positive coverage as 1st gens cephalosporins?
Yes → GP coverage is similar
Which cephalosporins are 3rd generation antibiotics that have good strep and GNR coverage? Do they have anaerobic coverage as well?
Ceftriaxone (IV), cefotaxime (IV), cefdinir (PO) , cefpodoxime (PO)
NO!
Which cephalosporin is a 3rd generation IV option that has limited GP coverage (no staph) and has coverage for PsA (Pseudomonas aeruginosa)
Ceftazidime (IV)
is a third-generation cephalosporin antibiotic used to treat infections caused by Gram-negative bacteria, particularly Pseudomonas aeruginosa.
What is the coverage of 3rd generation cephalosporins (ceftriaxone/cefotaxime/cefdinir/cefpodoxime) like?
Good coverage for Strep and Gram-negative rods (GNRs), but no anaerobic coverage

Which 3rd generation cephalosporin is active against Pseudomonas (GN) with limited GP coverage and NO Staph coverage.
Note: Staph is a GP🟣 bacteria
Ceftazidime (IV)

Which 4th generation cephalosporin is given IV? and has activity against PsA?
Cefepime (IV)
Which cephalosporins cover Pseudomonas (PsA)?
Ceftazidime (3rd gen) and cefepime (4th gen).
*BoTH IV admin
Which cephalosporins are alternatives for ENT infections in patients with a PCN allergy or resistant pathogens (S. pneumoniae, H. influenzae, M. catarrhalis)?
Cefdinir, Cefuroxime, Ceftriaxone
Which cephalosporin treats bacterial keratitis (Optho) caused by Staph aureus?
Cefazolin (and sometimes cefuroxime)
Which cephalosporin treats contact lens keratitis caused by Pseudomonas?
Ceftazidime
What is the main adverse effect of cephalosporins?
Hypersensitivity
Hypersensitivity = the possible immune reaction to the drug.
🔎 Hypersensitivity (Adverse Effect)
What it means: Your immune system “overreacts” to the drug, treating it like a dangerous foreign invader.
Types of reactions:
Mild: Rash, itching, hives
Moderate: Swelling (angioedema), fever
Severe (rare): Anaphylaxis → sudden drop in blood pressure, airway swelling, trouble breathing (medical emergency 🚨)
Why it happens: Cephalosporins contain a beta-lactam ring, similar to penicillins. Some people’s immune systems recognize it and attack.
What is the main contraindication for cephalosporins?
Cephalosporin allergy
Give examples of Carbapenems
Examples: Meropenem, Imipenem, Ertapenem
Do carbapenems have broad or narrow spectrum activity?
Broad-spectrum beta-lactam activity.
Which organisms do carbapenems cover?
Gram-positive, Gram-negative, and anaerobes.
Which carbapenem does not cover Pseudomonas aeruginosa (PsA)?
Ertapenem.
What is ertapenem’s main indication (when do we use this drug?”)
First line agent for ESBL E. coli infection what is this.
Extended-Spectrum Beta-Lactamase (ESBL)
💡 Quick memory tip:
Think ESBL = super bug, and Carbapenems (like ertapenem) = super strong antibiotics that can still kill it.
Which carbapenems provide Pseudomonas (PsA) coverage?
Which carbapenems DO NOT provide Pseudomonas (PsA) coverage?
Meropenem, imipenem, and doripenem.
Ertapenem – No PsA coverage
What is a major neurological & immune-related adverse effect that carbapenems can cause?
Seizures; Hypersensitivity reactions (allergic reactions)
In which patients should carbapenems be used cautiously or avoided?
Patients with seizure disorders.
Vancomycin belongs to which antibiotic class? Is this a Beta-Lactam?
Glycopeptide; No, does NOT have a beta-lactam ring.
Vancomycin (a Glycopeptide)
MOA: binds directly to D-Ala-D-Ala end of peptidoglycan precursors → prevents cell wall synthesis (different target than beta-lactams).
Still bactericidal, but different mechanism + structure.
What is the mechanism of action of vancomycin?
Vancomycin binds to the D-Ala–D-Ala terminus of peptidoglycan precursors, blocking polymerization and cross-linking needed for cell wall integrity.
It sticks to the “handles” (D-Ala–D-Ala) on the cell wall building blocks. This blocks the wall from being glued together, so the bacteria’s wall stays weak.
Is vancomycin bactericidal or bacteriostatic?
bactericidal
Vancomycin has activity against which types of organisms?
Aerobic and anaerobic Gram-positive bacteria.
Which major resistant bacteria is vancomycin effective against? Is vancomycin also effective against MSSA?
MRSA (Methicillin-resistant Staphylococcus aureus). Yes, it has activity against MSSA (Methicillin-sensitive Staphylococcus aureus).
Which genus of bacteria (besides Staph) is vancomycin active against?
Enterococcus spp.
Which serious GI infection is vancomycin used for (oral form)?
Clostridioides (Clostridium) difficile colitis.
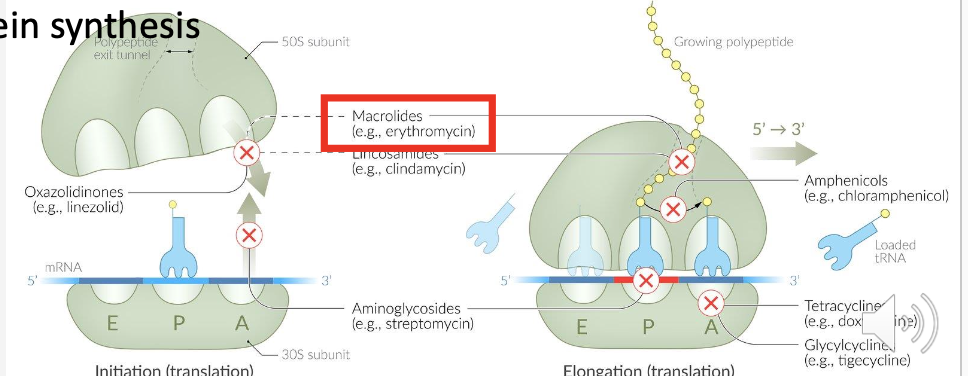
Which antibiotics are the main protein synthesis inhibitors? *both bacteriostatic
Macrolides (azithromycin, clarithromycin, erthyromycin)
Tetracyclines (doxy and mino)
Name two examples of tetracyclines.
Doxycycline and Minocycline.
How do tetracyclines block protein synthesis?
They prevent tRNA–AA from binding to the acceptor site on the ribosome-mRNA complex.
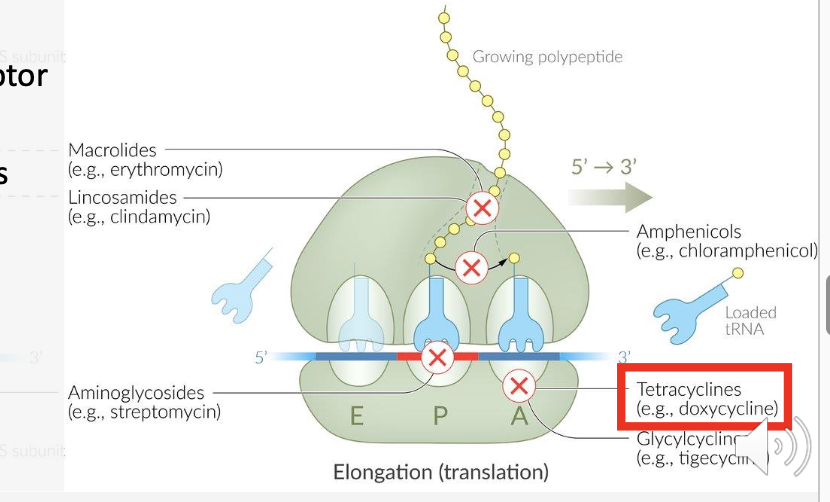
What ribosomal subunit do tetracyclines bind to?
The 30S ribosomal subunit.
Are tetracyclines bactericidal or bacteriostatic?
A: Bacteriostatic.
Why/How?
They don’t directly kill bacteria.
Instead, they stop bacteria from making proteins by blocking tRNA from binding at the ribosome.
Without proteins, bacteria can’t grow or multiply, but they don’t die immediately.
The body’s immune system then clears out the weakened bacteria.
💡 Quick memory tip:
“Static = Stop” (stop growth).
“Cidal = Kill.”
Which types of bacteria are tetracyclines (protein synthesis 30S ribosome inhibitors) active against?
Name some “atypical” organisms tetracyclines cover. (*x5)
Gram-positive and Gram-negative bacteria.
Protozoa, spirochetes, mycobacteria
What respiratory conditions can tetracyclines treat?
Atypical pneumonia and COPD exacerbations.
Which tick-borne illness is treated with tetracyclines?
Lyme disease.
Which ENT conditions can doxycycline treat?
AOM, sinusitis, periodontitis (Moraxella), Meibomian gland dysfunction.
Which ophthalmologic conditions can doxycycline treat?
Ocular rosacea and Chlamydial conjunctivitis.
What are the 3 main adverse effects of tetracyclines?
Tetracyclines:
Photosensitivity
esophageal irritation
tooth discoloration
Who should not take tetracyclines (contraindications)?
Pregnant women and children under 8 years old.
Name three examples of macrolides.
Azithromycin, Clarithromycin, Erythromycin.
What ribosomal subunit do macrolides bind to?
What key process do macrolides block? and How do macrolides inhibit bacterial growth?
Bind 50S ribosomal subunit;
Transpeptidation (peptide chain elongation).
They inhibit protein synthesis by blocking the ribosome.
What types of infections are macrolides (protein synthesis inhibitors) commonly used for?
Atypical pneumonia and ENT infections.
Which organisms causing atypical pneumonia are covered by macrolides? (*4)
Macrolides Treat:
Mycoplasma
Legionella
Chlamydia
Haemophilus influenzae
Which respiratory illness (whooping cough) is treated with macrolides?
Pertussis.
Which ENT infections can be treated with Azithromycin or Clarithromycin? (Macrolides)?
Rhinosinusitis or atypical ENT infections (e.g., Mycoplasma pharyngitis)
Which eye infections can be treated with Azithromycin drops?
bacterial conjunctivitis and trachoma (A chronic eye infection caused by Chlamydia trachomatis.)
What are the main adverse effects of macrolides?
Macrolides-
QT prolongation
GI upset
cholestatic hepatitis
Why do macrolides cause QT prolongation?
They interfere with cardiac ion channels, which can lead to dangerous arrhythmias.
In which patients are macrolides contraindicated?
Patients with QT prolongation or hepatic dysfunction.
Name three examples of aminoglycosides.
Gentamicin, Tobramycin, Amikacin.
Which ribosomal subunit do aminoglycosides bind to?
The 30S ribosomal subunit.
How do aminoglycosides cause faulty protein synthesis?
They cause misreading of mRNA, leading to incorrect amino acids and nonfunctional proteins.
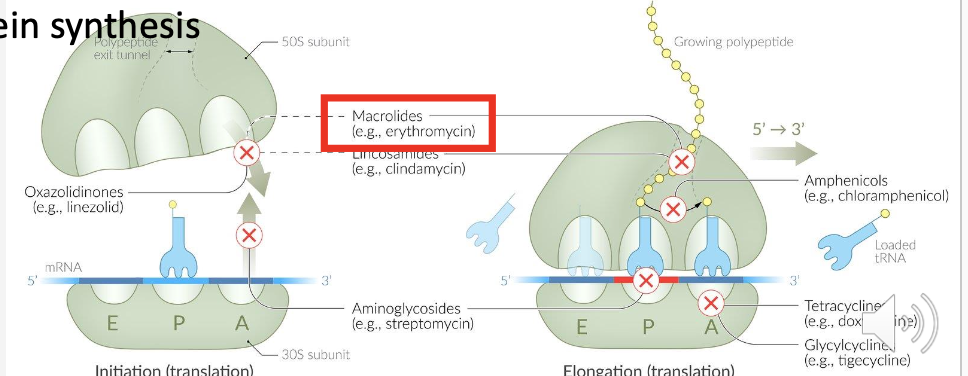
Besides misreading mRNA, what else do aminoglycosides inhibit?
Translocation.
Aminoglycosides: block translocation and cause misreading → junk proteins.
🔹 What is Translocation?
In protein synthesis, the ribosome moves along the mRNA strand like a train on a track.
Translocation = the step where the ribosome shifts forward by one codon (3 nucleotides).
This moves the growing protein chain into the next site on the ribosome so the next amino acid can be added.
Are aminoglycosides bactericidal or bacteriostatic?
Bactericidal.
What type of pneumonia are aminoglycosides used for?
Severe Gram-negative pneumonia.
Which difficult-to-treat pathogen is often targeted with aminoglycosides?
Pseudomonas infections.
Which aminoglycosides can be used for otitis externa?
Tobramycin and Gentamicin.
Which eye infections can be treated with Tobramycin drops/ointment? (aminoglycosides category)
Conjunctivitis and keratitis.
What are the main adverse effects of aminoglycosides?
Aminoglycosides-
Nephrotoxicity (They accumulate in the kidneys, damaging renal tubular cells.), ototoxicity (They accumulate in the inner ear, damaging auditory and vestibular hair cells.) and neuromuscular blockade
What conditions are contraindications for aminoglycoside use?
Renal failure and Myasthenia gravis (serious muscle weakness due to blocked ACh receptors.)
🚫 Why Aminoglycosides are Contraindicated
Aminoglycosides already block neuromuscular transmission.
In MG patients, this can make weakness much worse → even life-threatening breathing problems.
Fluoroquinolones (FQs)- DNA Killers:
Name three examples of fluoroquinolones.
Levofloxacin, Moxifloxacin, Ciprofloxacin
What enzyme do fluoroquinolones inhibit in Gram-negative bacteria?
DNA Gyrase (Topoisomerase II).
💡 Simple memory trick:
DNA gyrase = uncoiler
Block it → DNA stays tangled → bacteria can’t grow → they die.
What is the effect of inhibiting DNA Gyrase (Topo II)?
Blocks supercoil relaxation in Gram-negative bacteria.
What enzyme do fluoroquinolones inhibit in Gram-positive bacteria?
Topoisomerase IV.
What is the effect of inhibiting Topoisomerase IV?
Blocks chromosomal DNA separation during cell division in Gram-positive bacteria.
Are fluoroquinolones bactericidal or bacteriostatic?
They are mainly bactericidal, but can have bacteriostatic effects in some cases.
INFO: 🦠 Gram-Negative Bacteria VS. 🦠 Gram-Positive Bacteria
🦠 Gram-Negative Bacteria
Main Target: DNA Gyrase (Topoisomerase II)
Why? Gram-negative bacteria rely heavily on DNA gyrase to unwind their tightly packed circular DNA.
Effect: Blocking DNA gyrase prevents supercoil relaxation → replication stalls → cell death.
🔑 Example: Ciprofloxacin is especially strong against Gram-negatives (like Pseudomonas).
🦠 Gram-Positive Bacteria
Main Target: Topoisomerase IV
Why? Gram-positive bacteria depend more on Topo IV to separate newly replicated chromosomes.
Effect: Blocking Topo IV prevents chromosome separation during cell division → bacteria can’t divide → cell death.
🔑 Example: Levofloxacin and Moxifloxacin are stronger against Gram-positives (like Streptococcus pneumoniae).
What is ciprofloxacin mainly used for?
What are the limitations of ciprofloxacin?
Urinary tract infections (GU) and GI infections due to Gram-negative rods.
Increasing E. coli resistance, no Staphylococcus coverage, and poor Streptococcus coverage → not good for empiric therapy.
What is levofloxacin mainly used for?
Urinary and respiratory infections.
What Gram-positive organisms does levofloxacin cover?
MSSA, some coagulase-negative staphylococci (CoNS), Streptococcus pneumoniae, Streptococcus viridans, and Enterococcus faecalis.
What type of empiric therapy is levofloxacin good for?
Low-risk respiratory and GU infections.
Which anaerobe is covered by levofloxacin (fluoroquinolone)?
Peptostreptococcus (GP)
What is moxifloxacin mainly used for?
What is special about moxifloxacin’s spectrum?
Respiratory infections and GI infections.
It has the broadest anaerobic coverage of the fluoroquinolones, making it reliable for GI infections or aspiration pneumonia.
What are the limitations of moxifloxacin?
Limited anti-Pseudomonas activity and poor renal penetration.
Which fluoroquinolones are used for complicated sinusitis or resistant Strep pneumoniae?
Levofloxacin and Moxifloxacin.