AQA A-Level Physics Quantum phenomena
1/91
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
92 Terms
wavelength of light equation

What equation is used to determine the energy of a photon?
E = hf = hc/λ

What is threshold frequency?
The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface of a metal.
What is threshold wavelength?
The longest wavelength of incident electromagnetic radiation that would remove a photoelectron from the surface of a metal.
How do threshold frequency and wavelength relate to materials?
Threshold frequency and wavelength are properties of a material and vary from metal to metal.
work function
The work function Φ, or threshold energy, of a material, is defined as:
The minimum energy required to release a photoelectron from the surface of a metal
Consider the electrons in a metal as trapped inside an 'energy well' where the energy between the surface and the top of the well is equal to the work function Φ
A single electron absorbs one photon
Therefore, an electron can only escape from the surface of the metal if it absorbs a photon which has an energy equal to Φ or higher
Define the work function in the context of photoelectrons.
The work function Φ, or threshold energy, is the minimum energy required to release a photoelectron from the surface of a metal.
Explain the concept of an energy well in relation to electrons in a metal.
Electrons in a metal can be considered as trapped inside an 'energy well', where the energy between the surface and the top of the well is equal to the work function Φ.
Describe the relationship between photons and electrons in the context of the work function.
A single electron absorbs one photon, and it can only escape from the surface of the metal if it absorbs a photon with energy equal to the work function Φ or higher.
How does the work function affect the escape of electrons from a metal surface?
The work function determines the minimum energy required for an electron to escape; if the energy of the absorbed photon is equal to or greater than the work function, the electron can escape.
What is the significance of the energy of a photon in relation to the work function?
The energy of a photon must be equal to or greater than the work function for an electron to be released from the metal surface.
Why do different metals have different threshold frequencies
Different metals have different threshold frequencies and hence different work functions
Using the well analogy:
A more tightly bound electron requires more energy to reach the top of the well
A less tightly bound electron requires less energy to reach the top of the well
Photoelectric equation
hf = φ + KE_max, φ = work function: minimum energy required to release an electron from the metal surface
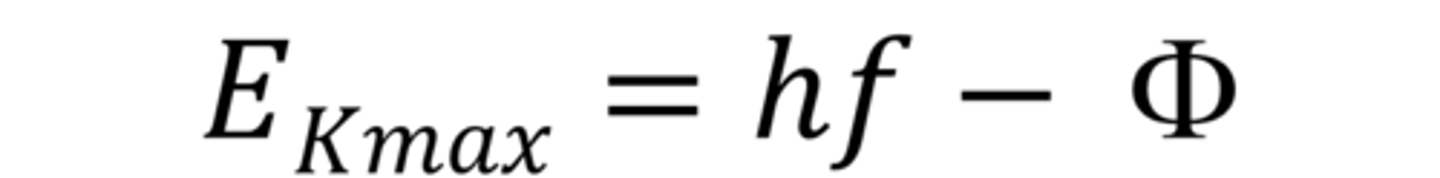
What is the relationship between the number of photoelectrons emitted per second and the intensity of incident radiation?
The number of photoelectrons emitted per second is proportional to the intensity of incident radiation.
If light is incident on a metal and photoelectric emission does NOT occur, what is the effect of increasing light intensity?
● If it is more intense then there would be more photons incident on the metal each second
● However each photon still carries the same amount of energy as before
● Therefore it still does not contain enough energy to liberate an electron
● No effect
Does photoelectric emission have a delay when incident radiation is directed at the surface?
No, photoelectric emission happens without delay.
What is Einstein's explanation of the photoelectric effect?
Light energy must come in packets called photons.
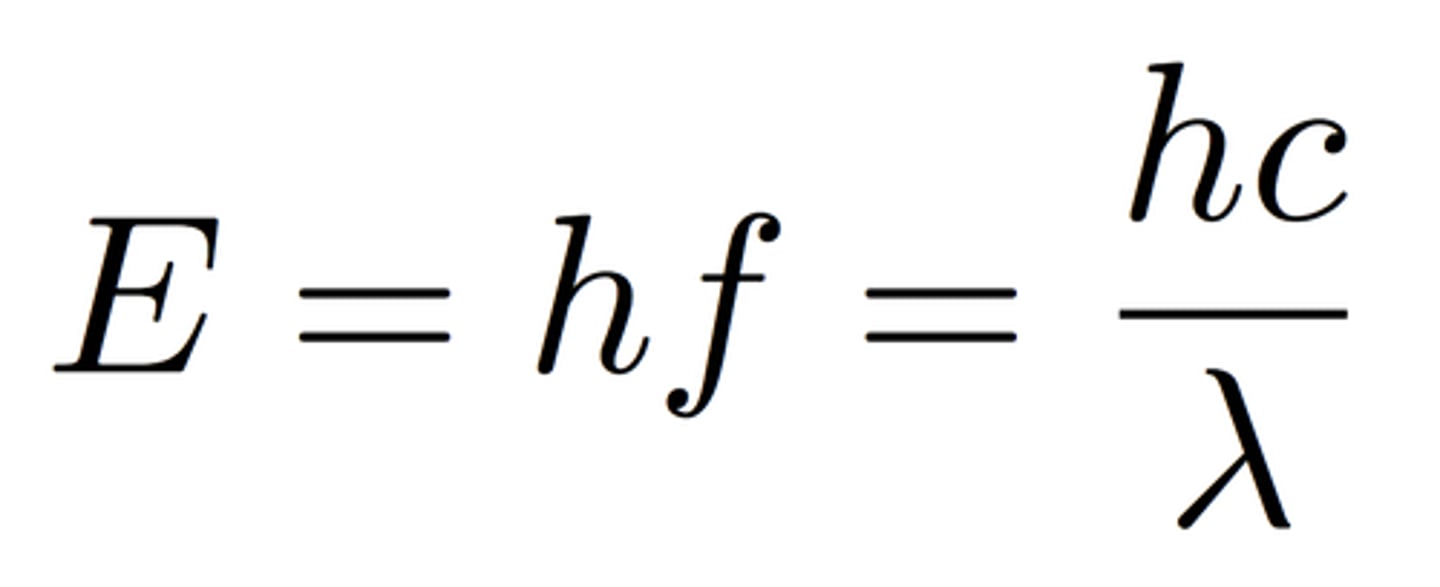
What happens when light is incident on a metal surface in the photoelectric effect?
An electron at the surface absorbs a single photon and gains energy equal to E=hf.
What is the minimum energy needed for an electron to escape from a metal surface?
The energy gained from a single photon must exceed the work function of the metal.
What happens to the excess energy gained by a photoelectron?
It becomes the kinetic energy of the photoelectron.
What is stopping potential?
The minimum potential difference required to stop photoelectric emission.
What happens to the maximum kinetic energy of emitted electrons at stopping potential?
It is 0 because each emitted electron must do extra work.
Conduction electrons
Electrons in a metal that are free to move around in the metallic crystal lattice.
What is the work function of a metal?
The minimum energy required by a conduction electron to escape from the metal surface when the metal is at 0 potential.
What happens when a conduction electron absorbs a photon?
The kinetic energy of the electron will be equal to the energy of the photon.
What occurs if the energy of a photon exceeds the work function of a metal?
Conduction electrons can leave the metal.
What happens to an electron that does not leave the metal after absorbing energy?
It collides repeatedly with other electrons and positive ions, losing extra kinetic energy.
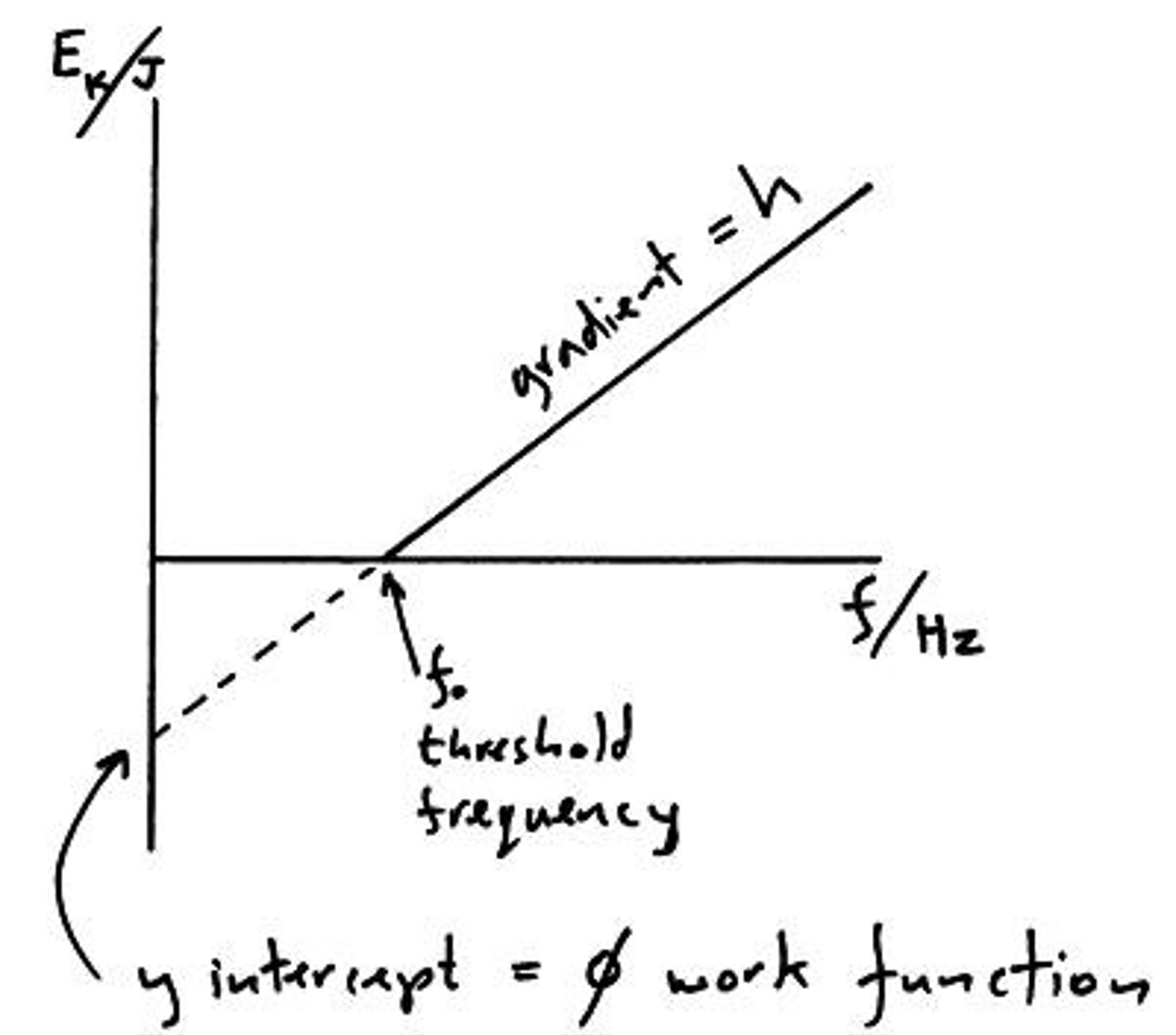
graph of Ek against frequency
gradient = Planck constant
y intersect = work function
x intersect = threshold frequency
What is a vacuum photocell?
A glass tube that contains a metal plate (cathode) and a smaller metal electrode (anode).
What happens when light at or above threshold frequency is shone on a vacuum photocell?
Electrons from the photocathode are attracted to the anode.
How can a vacuum photocell be used in a circuit?
It can be connected in a circuit to measure the photoelectric current.
what is an ion
an atom or molecule with a net electric charge due to the loss or gain of one or more electrons.
ionisation
Any process in which atoms become charged, by removing or adding electrons
electron volt (eV)
Unit of energy equal to 1.6 x 10^-19 joules, used to quantify energy transfers on the atomic scale
How does excitation energy compare to ionisation energy?
Excitation energy is always less than ionisation energy.
ground state
The lowest energy state of an atom
What happens when an atom in ground state absorbs energy
When an atom in ground state absorbs energy, one of electrons move to a higher energy level, so atom is now in excited state
Excited state
a state in which an atom has more energy than it does at its ground state
energy levels of atom
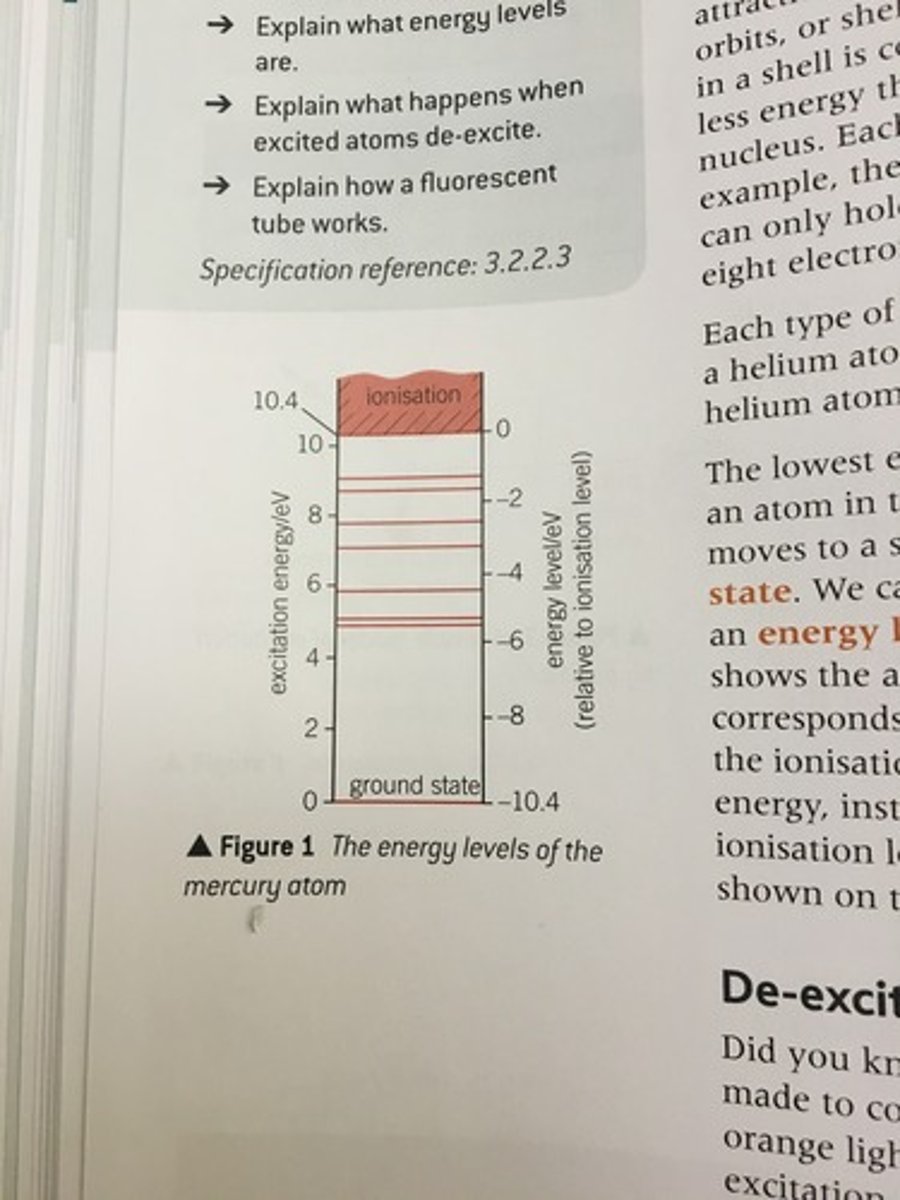
excitation energy
the energy required to move an electron from a lower energy level to a higher energy level
What happens in excitation by collision
When a free electron collides with an atom, it can transfer energy to one of the atom's electrons.
How does electron energy impact excitation
If the incoming electron has energy equal to or greater than the excitation energy, the atomic electron jumps to a higher energy level.
What happens to the atom after it is excited
After excitation, the atom is in an unstable state and eventually returns to the ground state by emitting photons (light).
What occurs during an elastic collision involving an electron?
The colliding electron simply bounces off the atom without causing excitation.
Why is there no energy transfer to the atomic electron during an elastic collision?
Energy levels in an atom are quantized, so the atomic electron can only be excited if the incoming electron provides at least the required excitation energy.
What happens to the free electron's energy after an elastic collision?
The incoming electron continues moving, possibly changing direction, but with nearly the same energy as before.
What is the result regarding photon emission during an elastic collision?
Since no excitation occurs, the atom does not emit any photons.
What happens when an atom absorbs energy?
The atom excites an electron to a higher level.
How long does an electron remain in the excited state?
Around 10−9 seconds.
What occurs when an electron returns to a lower energy level?
It releases energy as a photon.
What does the emitted photon correspond to?
The energy difference between the levels.
What is multi-step de-excitation?
An electron falls in steps through intermediate energy levels instead of returning directly to the ground state.
What is released at each step of multi-step de-excitation?
A photon with a different frequency.
What do the photons released during de-excitation produce?
Spectral lines in an emission spectrum.
How do fluorescent tubes work?
Electrons collide with mercury atoms, exciting them, which then emit ultraviolet (UV) photons.
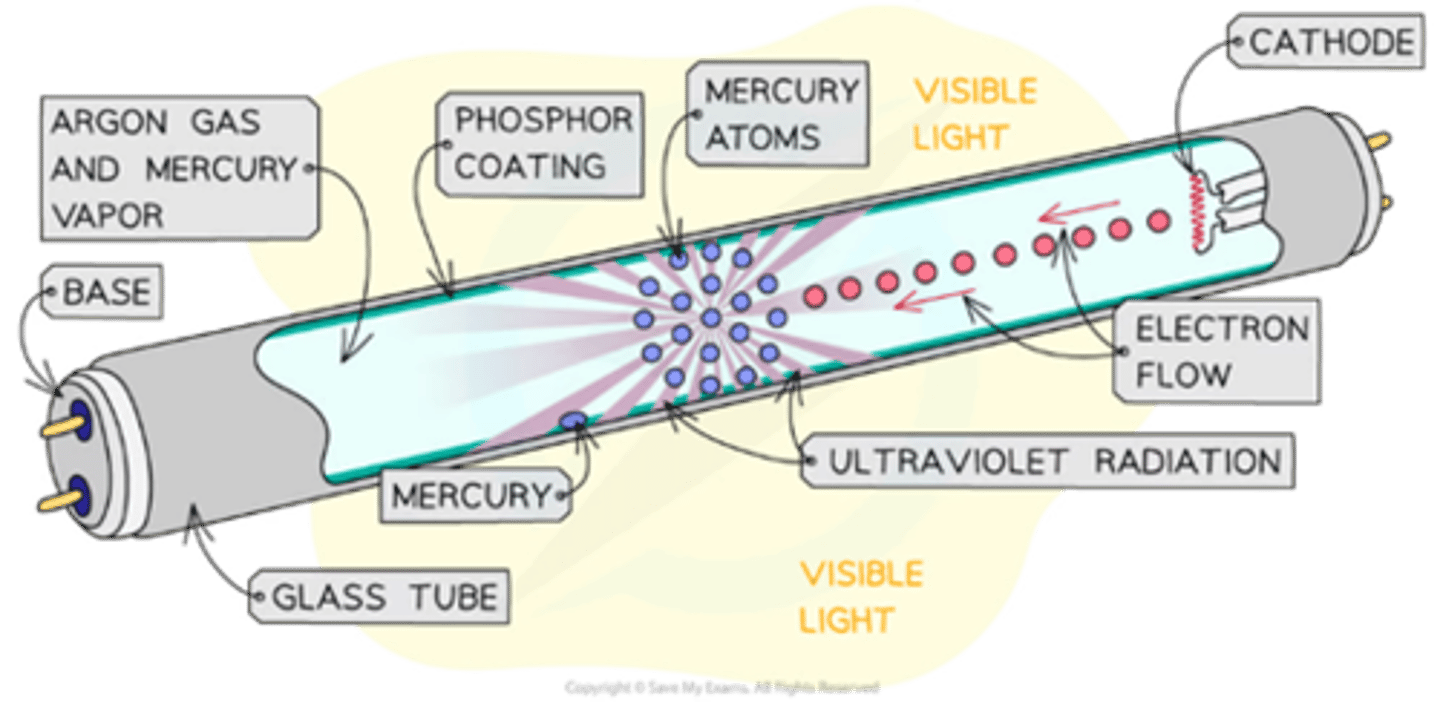
What happens to UV photons in fluorescent tubes?
They are absorbed by a phosphor coating, which then emits visible light.
What do excited neon atoms release in neon lights?
Photons of specific wavelengths, producing red light.
What is unique about each element's energy levels?
Each element has a unique set of energy levels.
What do emitted photons create in an emission spectrum?
Discrete lines.
Energy of emitted photon
hf = E₁ - E₂ (two energy levels)

What happens when an atom absorbs a photon with the exact energy needed?
Excitation occurs, promoting an electron to a higher energy level.
What condition must be met for a photon to be absorbed by an atom?
The photon's energy must exactly match the energy gap between two energy levels.
What happens if the photon energy is too low?
The photon passes through without being absorbed.
What happens if the photon energy is too high?
The electron may be ionized, meaning it is removed from the atom entirely.
What occurs when white light passes through a gas?
Specific frequencies are absorbed, corresponding to excitation energies.
What do the missing frequencies in an absorption spectrum represent?
They appear as dark lines in the spectrum.
What is the basis of astronomical spectroscopy?
It involves analyzing the light emitted and absorbed by stars to determine their composition.
What gas is used in a fluorescent tube?
Mercury gas at low pressure
What happens to gas in a fluorescent tube when current flows through it?
The gas is ionised and excited as electrons collide with atoms.
What do mercury atoms emit when they de-excite?
UV photons
What does the fluorescent coating on the inner wall of a tube do?
It absorbs UV photons and causes excitation.
What happens when excited electrons in the fluorescent coating de-excite?
They emit photons in the visible range.
What is a line spectrum?
A line spectrum is when light is shown through a prism and produces bands of light.
What is a continuous spectrum?
A continuous spectrum is the emission of a continuous range of frequencies.
What causes each line in a line spectrum?
Each line is due to light of a certain color and of a certain wavelength.
What is true about the photons that produce each line in a line spectrum?
Photons that produce each line all have the same energy, which is different from the energy of photons that produce other lines.
What happens when an atom de-excites?
A photon is emitted when one of its electrons moves to an inner shell.
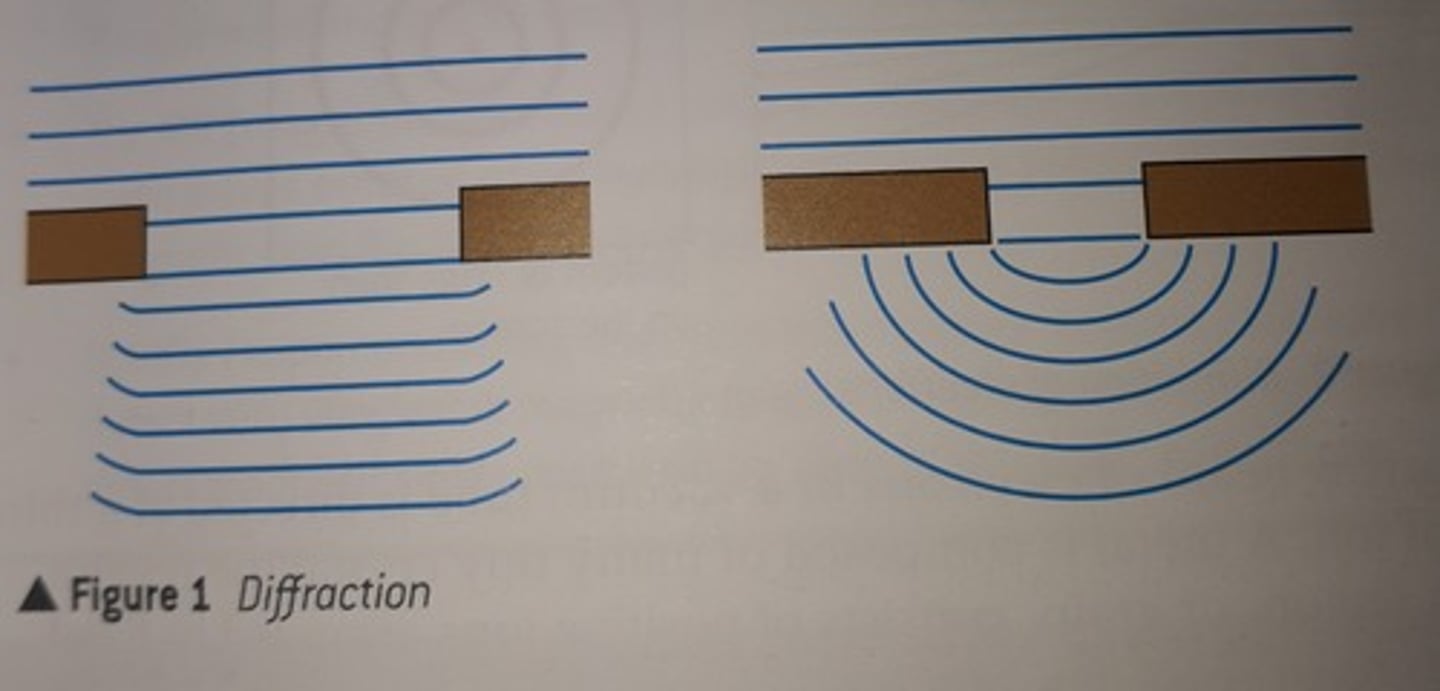
Wave-like nature of light
Observed when diffraction of light takes place.
Particle-like nature of light
Demonstrated by the photoelectric effect
Matter waves
wave characteristics of material particles
de Broglie Hypothesis
All particles can behave like waves whose wavelength is given by λ = h/p where h is Planck's constant and p is the momentum of the particle.
de Broglie wavelength properties
Wave-like behaviour of matter particle is characterised by a wavelength , which is related to the momentum of the particle in the equation
de Broglie wavelength equation
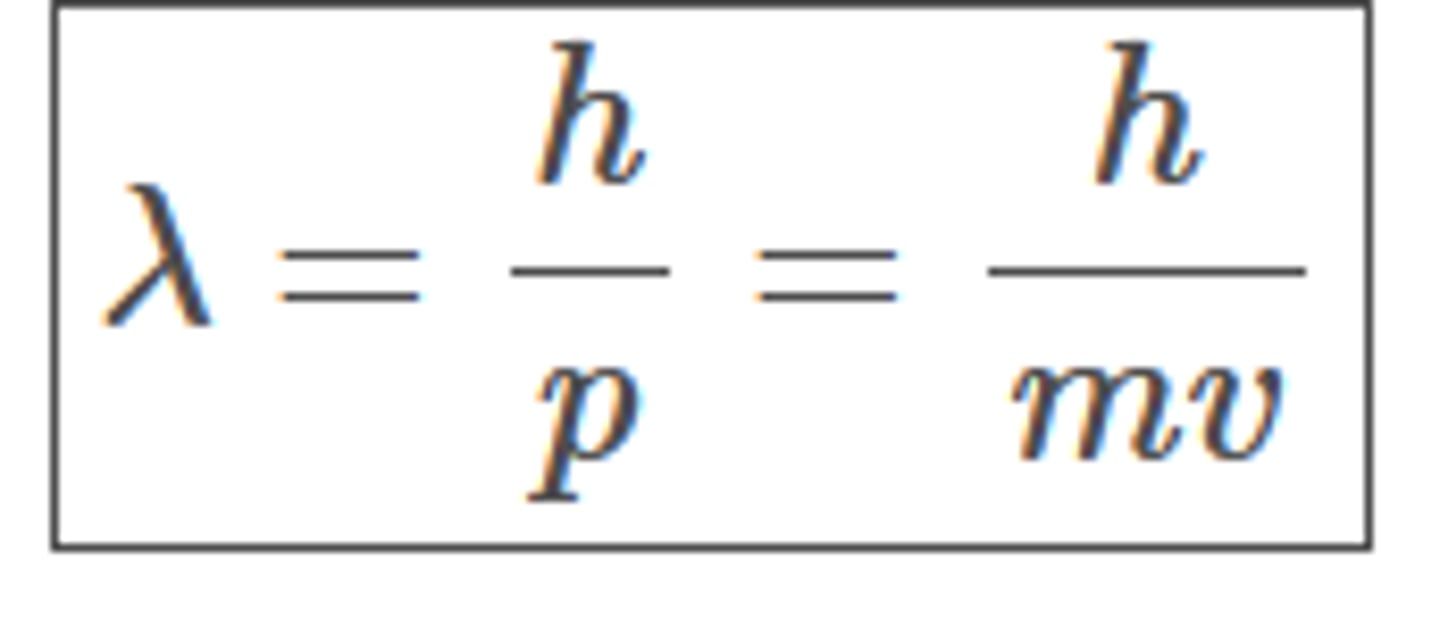
What happened in the Davisson-Germer Experiment (1927)?
Electrons were fired at a nickel crystal.
What was the result of the Davisson-Germer Experiment?
A diffraction pattern was observed, confirming wave-like behavior.
What conclusion was drawn from the Davisson-Germer Experiment?
Electrons act as waves, supporting matter waves.
What happened in G.P. Thomson's Experiment (1927)?
Electrons passed through a thin metal foil and created an interference pattern.
What conclusion was drawn from G.P. Thomson's Experiment?
Electrons exhibit wave-like interference, further proving wave-particle duality.
What is wave-particle duality?
Electrons behave as waves when diffracted, but as particles in interactions.
How do electrons explain their orbits in atoms?
Electrons form standing waves in quantized energy levels.
What is a practical application of wave-particle duality in microscopy?
Electron microscopes use shorter de Broglie wavelength for higher resolution.
What is a practical application of wave-particle duality in crystallography?
Studying atomic structures using diffraction.