Chemistry IGCSE - Atoms, elements and compounds foundation
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
Elements
A substance made up of only 1 type of atom
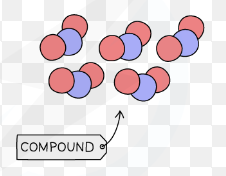
Compounds
A substance made up of 2 or more different types of atoms
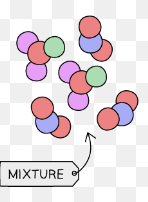
Mixture
contains 2 or more elements/compounds that are not chemically bonded
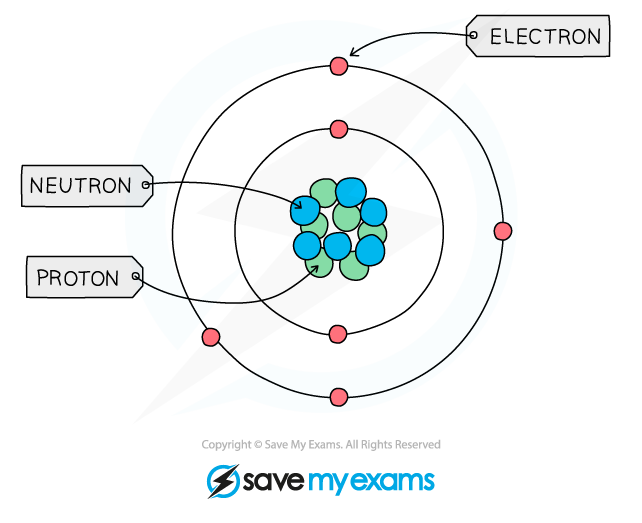
Describe the structure of the atom
a central nucleus containing neutrons and protons, surrounded by electrons in shells
Relative charge and relative mass of a proton
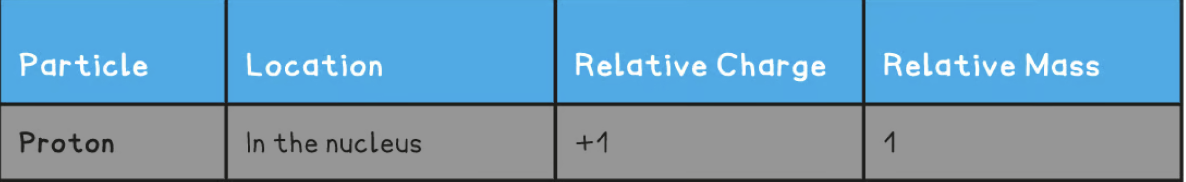
Relative charge and relative mass of a neutron
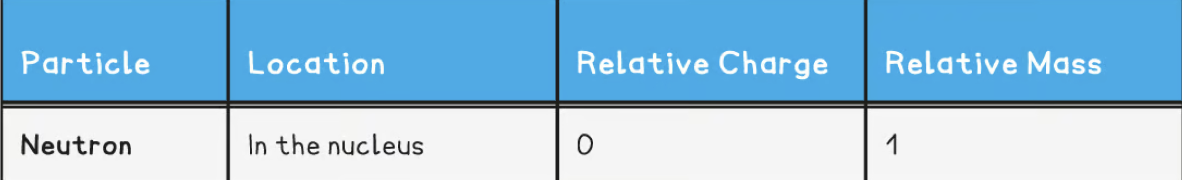
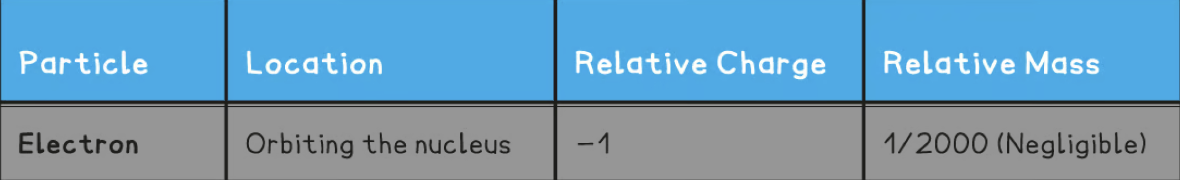
Relative charge and relative mass of an electron
Proton number/atomic number
number of proton in the nucleus of an atom
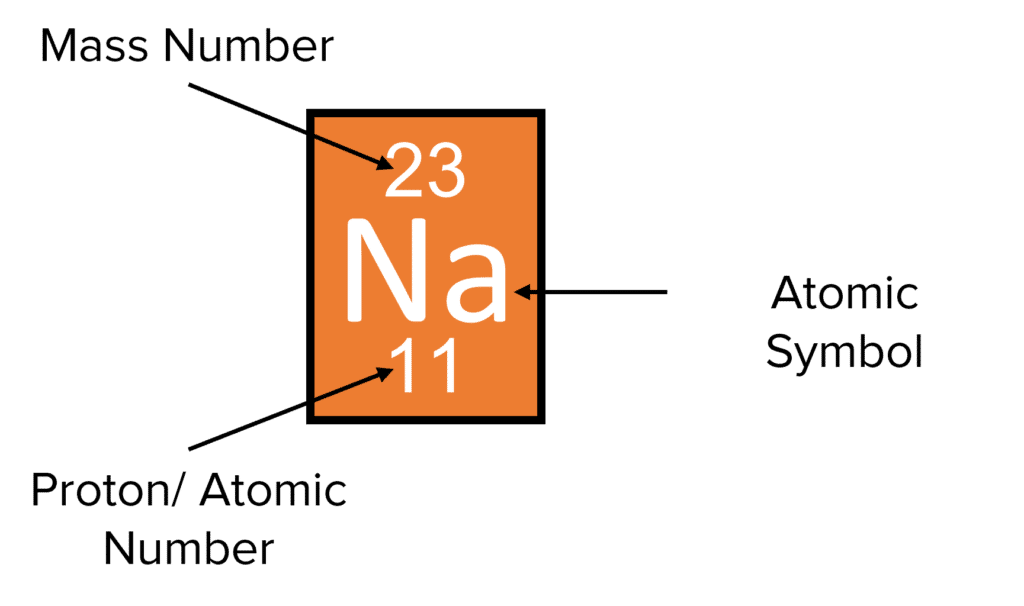
Mass number/nucleon number
the total number of protons and neutrons in the nucleus of an atom
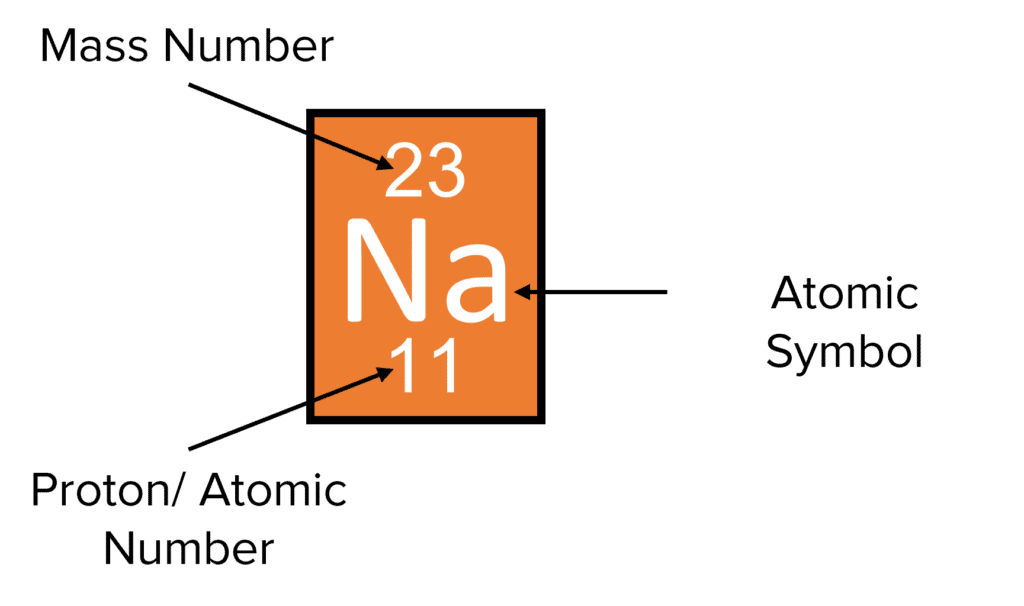
Electronic configuration
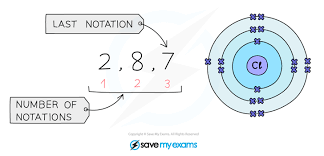
Group VIII (8th)
full outer shell
connection between no. of valence shell electrons and group no.
the no. of outer-shell electrons = group no. in Groups I to VII
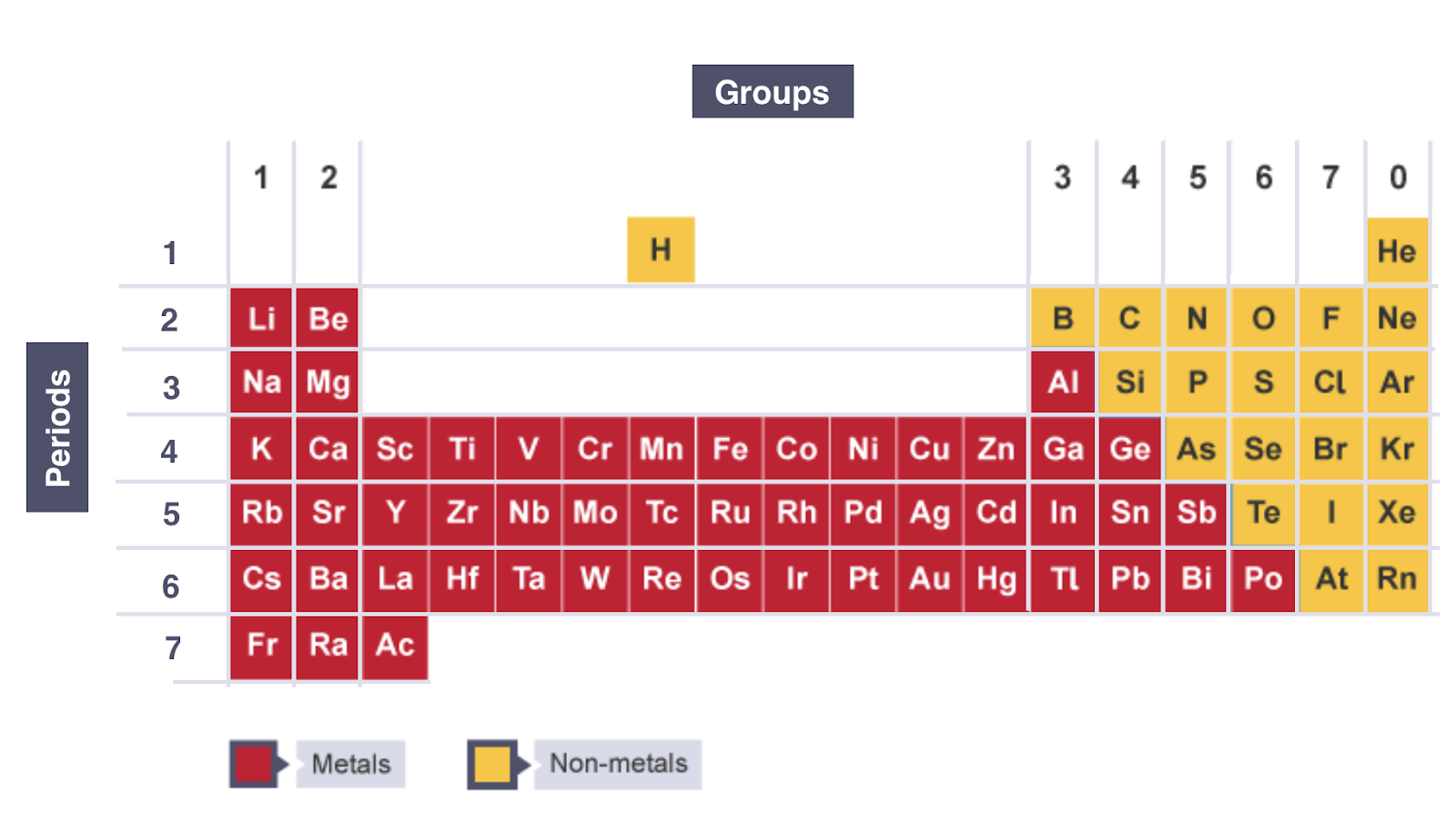
connection between no. of occupied electron shells and period no.
the no. of occupied electron shells = period no.
Isotope
different atoms of the same element that have the same no. of protons but different no. of neutrons
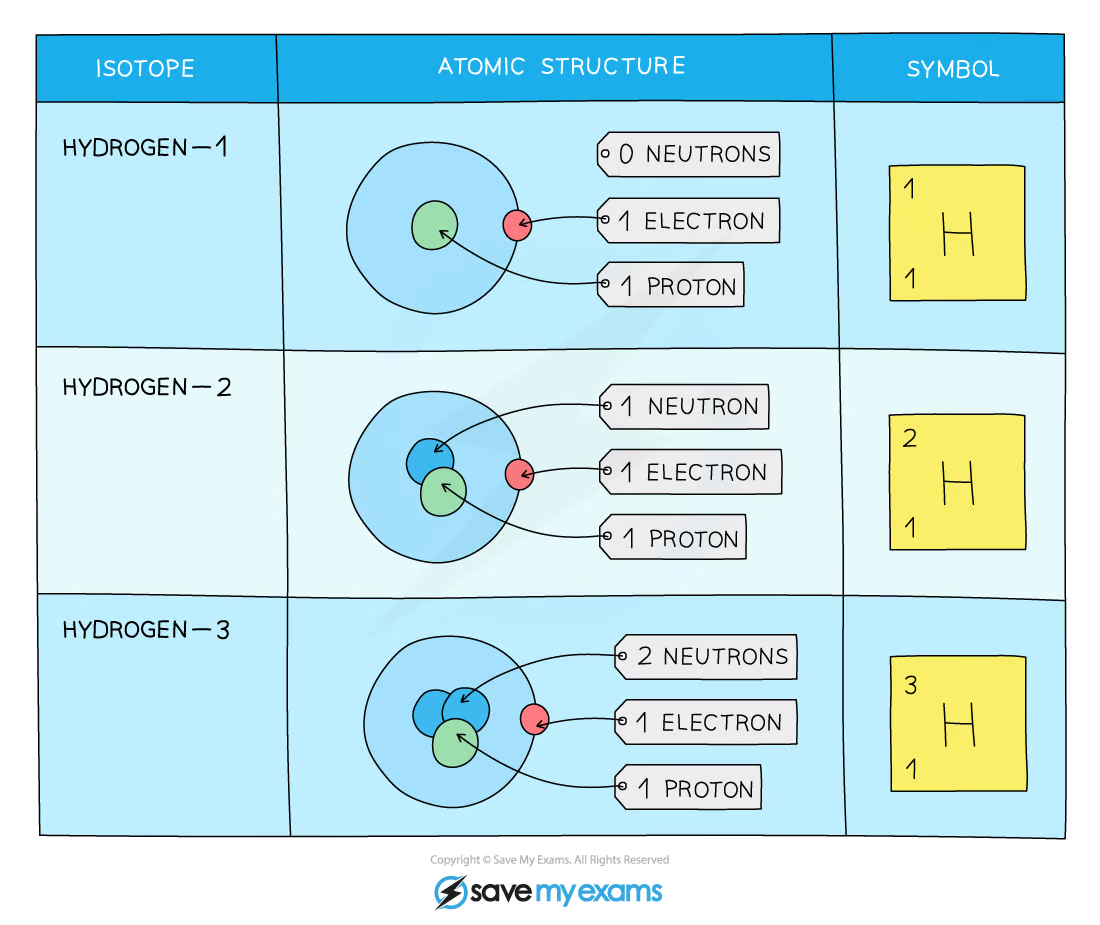
Isotopes with similar chemical properties
Isotopes of the same element have the same chemical properties → same no. of electrons → same electronic configuration
Alloy
a mixture of two or more metals or metal with a non-metal