How Fast
1/18
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
Define rate
change in concentration of reactant or product with time
how to work out rate of reaction and units
change in concentration of reactant or product / time taken for the change to take place
moldm^-3 s^-1
When is the highest rate of reaction
at t=0
rate is highest when gradient is steepest
state and explain how the rate of reaction changes with time
*the rate of reaction decreases with time
*concentration of HCl(aq) decreases with time
*there are less particles per unit volume
*there are less successful collisions per unit time
how to work out the overall order of a reaction
add the orders
e.g. add the powers in the rate equation
what is the rate equation
Rate = k[A]^m[B]^n where m and n are the orders with respect to reactants A and B respectively, [A] and [B] are the concentrations of A and B respectively and k is the rate constant
what are the units of concentration
mol dm^-3
what are the units of rate
mol dm^-3 s^-1
what is the relationship between first order k and half life?
k = ln2/half life
what is the order of a reactant if it has no effect on rate
zero order
what is the link between the rate equation and the RDS
rate equation only includes species involved in the RDS
what is significant about half lives in first order reactions
constant half life
what is the slowest step in a mechanism called
rate determining step
what is the order of a reactant if doubling the conc leads to rate x4
second order
what is the order of a reactant if doubling the conc doubles the rate
first order
which equation links the rate constant and the activation energy
Arrhenius equation
what is meant by the half life of a reactant
the time taken for the concentration of a reaction to decrease to half its original value
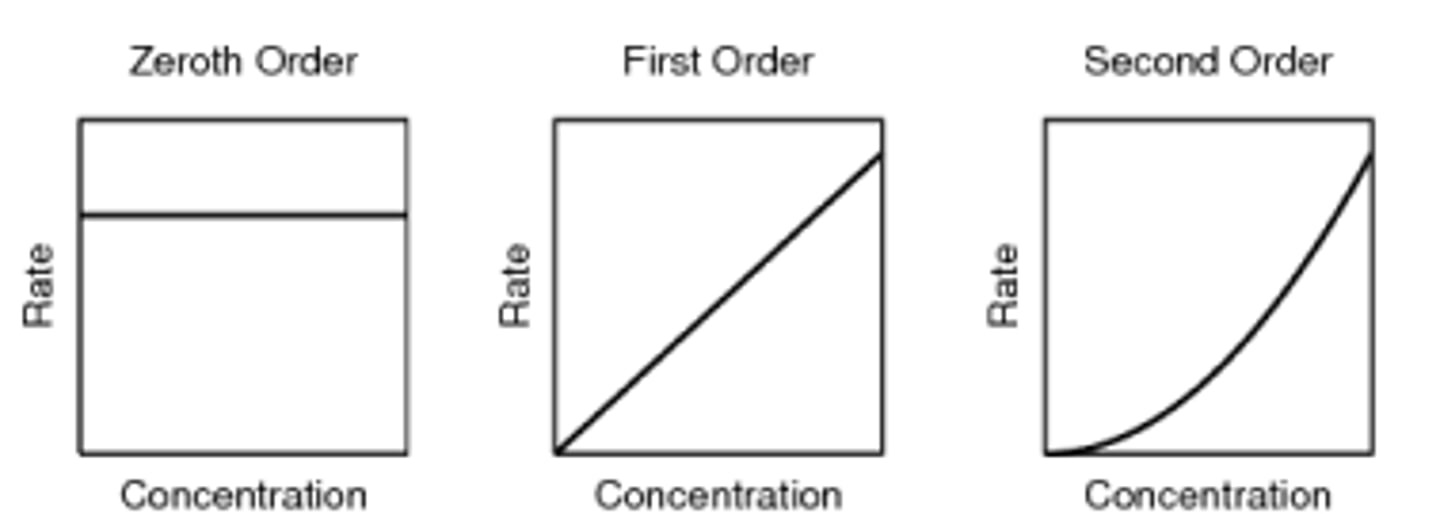
what do the rate against concentration graphs look like for the zero, first and second orders
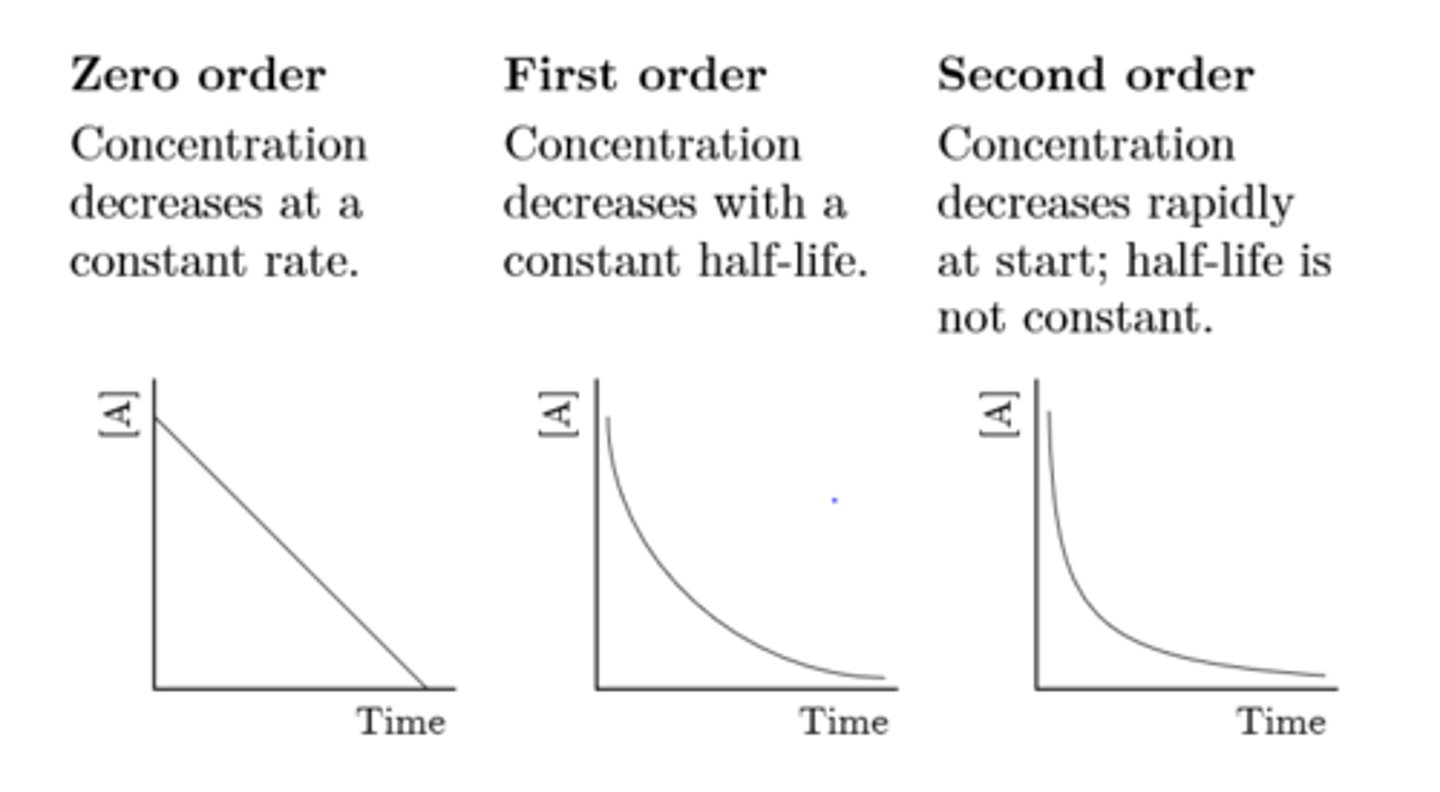
what do the concentration against time graphs look like for the zero, first and second order
zero order - half life decreases with time
first order- constant half life
second order - half life increases with time