18 - carboxylic acids and derivatives
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
carboxylic acids
compounds with a -COOH (carboxyl functional group)
They can be prepared by a series of different reactions
they include a carbonyl and hydroxyl group
they are isomeric with esters: -RCOOR’
how can carboxylic acids be formed
oxidation of primary alcohols and aldehydes
hydrolysis of nitriles
hydrolysis of esters
Oxidation of primary alcohols & aldehydes
Carboxylic acids can be formed from the oxidation of primary alcohols and aldehydes by either acidified K2Cr2O7 or acidified KMnO4 and reflux
The oxidising agents themselves get reduced causing the solutions to change colour
In K2Cr2O7 the orange dichromate ions (Cr2O72-) are reduced to green Cr3+ ions
In KMnO4 the purple manganate ions (MnO4-) are reduced to colourless Mn2+ ions
Oxidation of primary alcohols and aldehydes
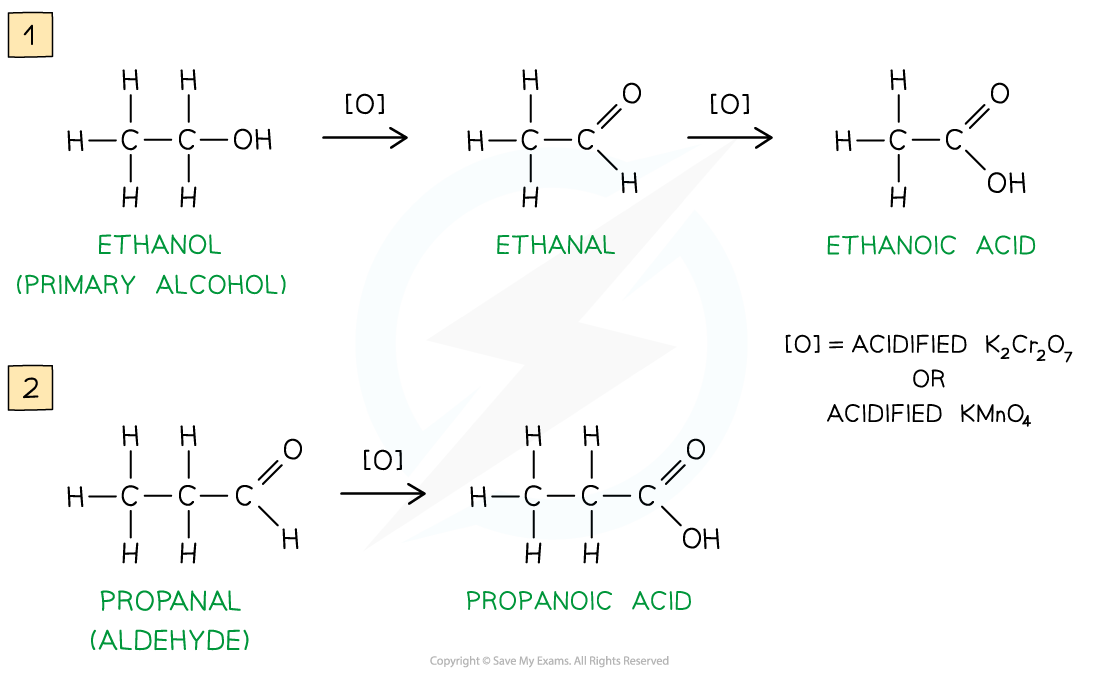
hydrolysis of nitriles
Carboxylic acids can also be prepared from the hydrolysis of nitriles using either dilute acid or dilute alkali followed by acidification
Hydrolysis by dilute acid results in the formation of a carboxylic acid and ammonium salt
Hydrolysis by dilute alkali results in the formation of a sodium carboxylate salt and ammonia; Acidification is required to change the carboxylate ion into a carboxylic acid
both ways are heated under reflux
The -CN group at the end of the hydrocarbon chain is converted to a -COOH group
Hydrolysis of nitriles

hydrolysis of esters
Esters are formed from the condensation reaction between an alcohol and a carboxylic acid
Hydrolysis of esters by dilute acid or dilute alkali and heat followed by acidification will reform the alcohol and the carboxylic acid
Hydrolysis by dilute acid is a reversible reaction where an equilibrium is established
Hydrolysis by dilute alkali is an irreversible reaction as all the ester is broken down to form a sodium carboxylate salt and an alcohol; acidification is required to change the carboxylate ion into a carboxylic acid
Hydrolysis of esters

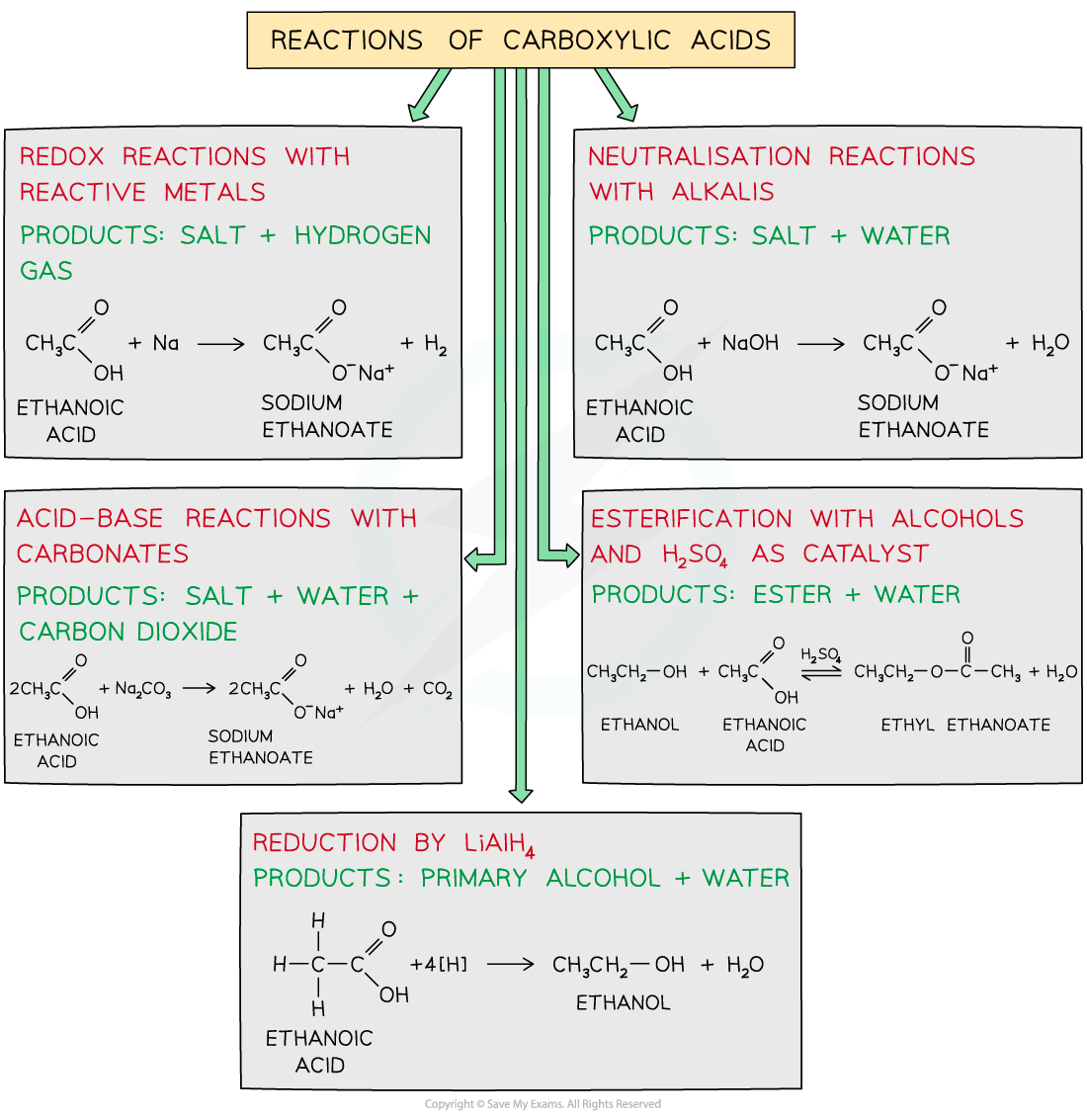
reactions of carboxylic acids
Carboxylic acids are weak acids as they do not completely dissociate in water
This means that the position of the equilibrium lies to the left and that the concentration of H+ is much smaller than the concentration of the carboxylic acid
The solution has a pH value of less than 7
Example dissociation of a carboxylic acid

Carboxylic acids are weak acids that do not fully dissociate in water, the position of the equilibrium lies to the left
Carboxylic acids are reactive compounds which can undergo many types of reactions including:
Redox reactions with reactive metals
Neutralisation reactions with alkali
Acid-base reactions with carbonates
Esterification with alcohols
Reduction by LiAlH4
different reactions of carboxylic acids
esters
ompounds with an -COOR functional group and are characterised by their sweet and fruity smells
They are prepared from the condensation reaction between a carboxylic acid and alcohol with concentrated H2SO4 as catalyst
This is also called esterification
The first part of the ester’s name comes from the alcohol and the second part of the name comes from the carboxylic acid
E.g. Propanol and ethanoic acid will give the ester propyl ethanoate

hydrolysis of esters (pt 2)
Esters can be hydrolysed to reform the carboxylic acid and alcohol by either dilute acid or dilute alkali and heat
When an ester is heated under reflux with dilute acid (eg. sulfuric acid) an equilibrium mixture is established as the reaction is reversible
Example acid hydrolysis of an ester

Ester hydrolysis by dilute acid is a reversible reaction forming carboxylic acid and alcohol
However, heating the ester under reflux with dilute alkali (eg. sodium hydroxide) is an irreversible reaction as the ester is fully hydrolysed
This results in the formation of a sodium carboxylate salt which needs further acidification to turn into a carboxylic acid
The sodium carboxylate (-COO-) ion needs to get protonated by an acid (such as HCl) to form the carboxylic acid (-COOH)
Example alkaline hydrolysis of an ester

Ester hydrolysis by dilute alkali is an irreversible reaction forming a sodium carboxylate salt and alcohol
physical properties of carboxylic acids
1. Hydrogen bonding → High boiling points
COOHs form extensive hydrogen bonds with each other due to:
The –OH group (hydrogen donor)
The C=O group (hydrogen acceptor)
This leads to high boiling points compared to similar-sized molecules (e.g. aldehydes, ketones, alcohols).
2. Solubility in water
Short-chain COOHs (e.g. methanoic acid, ethanoic acid) are very soluble in water because they form hydrogen bonds with water molecules.
Longer-chain COOHs become less soluble as the hydrophobic hydrocarbon tail dominates.
3. Smell
Many carboxylic acids have a sharp, sour, or vinegar-like smell (e.g. ethanoic acid = vinegar).
Larger ones may have an unpleasant or rancid smell.
chemical properties of carboxylic acids
1. Acidity
COOHs are weak acids:
They partially dissociate in water:
R–COOH⇌R–COO⁻+H⁺R–COOH⇌R–COO⁻+H⁺
The negative charge on the carboxylate ion (R–COO⁻) is stabilised by resonance, making them more acidic than alcohols.
2. Reactions with metals (e.g. Mg, Zn)
Form a salt and hydrogen gas:
2CH3COOH+Mg→(CH3COO)2Mg+H22CH3COOH+Mg→(CH3COO)2Mg+H2
3. Reactions with bases (e.g. NaOH)
Form a carboxylate salt and water:
CH3COOH+NaOH→CH3COONa+H2OCH3COOH+NaOH→CH3COONa+H2O
4. Reactions with carbonates (e.g. Na₂CO₃)
Form a salt, water, and CO₂ gas:
2CH3COOH+Na2CO3→2CH3COONa+H2O+CO2↑2CH3COOH+Na2CO3→2CH3COONa+H2O+CO2↑
5. Esterification with alcohols (with H₂SO₄ catalyst)
Form an ester and water:
CH3COOH+CH3CH2OH→H⁺CH3COOCH2CH3+H2OCH3COOH+CH3CH2OHH⁺CH3COOCH2CH3+H2O
6. Reduction (only in A2, not AS)
For reference only: COOHs can be reduced to primary alcohols using LiAlH₄ in dry ether.
This is not required at AS level in CAIE.
physical properties of esters
1. Boiling points
Esters have moderate boiling points:
Lower than carboxylic acids and alcohols (no hydrogen bonding between ester molecules).
Higher than alkanes (they do have dipole–dipole interactions from the polar C=O bond).
2. Solubility in water
Small esters (like ethyl ethanoate) are slightly soluble in water:
They can form hydrogen bonds with water, but not between themselves.
Larger esters become insoluble due to their longer non-polar hydrocarbon chains.
3. Smell
Esters have sweet, fruity smells:
Used in perfumes and flavourings (e.g. banana, pineapple, rum, etc.).
chemical properties of esters
1. Hydrolysis (reaction with water or base)
This is the main chemical reaction you need to know at AS level.
(a) Acid hydrolysis (reversible)
Ester + water ⇌ carboxylic acid + alcohol
Requires dilute H⁺ (e.g. HCl) and heat.
Example:
CH3COOCH2CH3+H2O→H⁺, heatCH3COOH+CH3CH2OHCH3COOCH2CH3+H2OH⁺, heatCH3COOH+CH3CH2OH
(b) Alkaline hydrolysis (saponification) (irreversible)
Ester + NaOH → carboxylate salt + alcohol
Example:
CH3COOCH2CH3+NaOH→CH3COONa+CH3CH2OHCH3COOCH2CH3+NaOH→CH3COONa+CH3CH2OH
Note: This is preferred in industry because it goes to completion.
🔧 Preparation of Esters (Esterification)
Carboxylic acid + alcohol → ester + water
Requires concentrated H₂SO₄ as a catalyst and heat.
This is a reversible condensation reaction.
Example:
CH3COOH+CH3CH2OH→H⁺CH3COOCH2CH3+H2OCH3COOH+CH3CH2OHH⁺CH3COOCH2CH3+H2O