3.1.3 Bonding
1/71
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
72 Terms
What is a molecule?
2 or more non-metal atoms covalently bonded
What is a compound?
Two or more elements chemically bonded
What is an element?
Made up of the same atom
What is an ionic bond?
Electrostatic attraction between oppositely charged ions
What is a covalent bond?
Shared pair of electrons where each atom donates 1 electron to the pair
What is a co-ordinative (dative covalent) bond?
Shared pair of electrons where one atom donates both electrons
What is a metallic bond?
Electrostatic attraction between delocalised electrons and positive ions
What is electronegativity?
The power of an atom to attract a shared pair of electrons in a covalent bond
What is a solute?
A substance which is dissolved in a solvent
What is a solvent?
A substance which dissolves a solute
What happens in metals?
Positive metal ions are packed close together in layers in a regular way. This 3D arrangement is called a giant metallic lattice
What does delocalised mean?
Free to move throughout the structure
How should metallic bondings be drawn?
Put layers of the positive metal ions and put delocalised electron (amount lost) in between them or on the outside
In terms of metallic bonding, how would a diagram of the structure of magnesium differ from the one for sodium?
Mg2+ ions are slightly smaller with a higher charge. There are twice as many delocalised electrons
Across period 3 and down group 2, What happens to the melting points of metals?
Increases across period 3
Generally (magnesium deviates from this trend) decreases down group 2
Explain why aluminium has a higher melting point than sodium
Al3+ ions are smaller and more highly charged than Na+ and so are more strongly attracted to the delocalised electrons, of which there are more
Explain why calcium has a higher melting point than strontium?
Ca2+ ions are smaller than Sr2+ ions and so are more strongly attracted to the delocalised electrons
How does a giant metallic lattice have a high melting point?
Due to having strong electrostatic attractions between the positive ions and the delocalised electrons
How does a giant metallic lattice conduct electricity?
When liquid or solid because delocalised electrons can flow through structure and carry current
How is a giant metallic lattice strong?
Due to having strong electrostatic attractions between the positive ions and the delocalised electrons
What does malleable and ductile mean?
Malleable - substance can be pressed into shape without breaking
Ductile - substance is able to be drawn out into a thin wire
Why is a giant metallic lattice malleable and ductile?
Layers of ions in the giant metallic lattice can slide over one another into new positions, without disrupting the metallic bond
What are the important things to know of ionic bondings?
Boron (G3) doesn’t form ions
In G4, C and Si doesn’t usually form ions
In G4, In tin, Sn+4 ion is most stable, but Sn2+ is also seen
In G4, Lead compounds generally contain Pb2+ ion, but some contain Pb4+
Transition elements form more than one stable ion
What is the difference between sulfate IV and VI
Sulfate IV → SO32-
Sulfate VI → SO42-
What kind of structure do ionic compounds have?
Giant ionic lattice structure, held together by strong electrostatic forces. negative and positive ions alternate in the lattice. Each ion is surrounded by oppositely charged ions in all directions
What is the melting point of a giant ionic lattice?
High due to strong electrostatic attractions between oppositely charged ions
What is the electrical conductivity of a giant ionic lattice?
When solid: Insulator, ions are in fixed positions
When dissolved or molten: Conductor as ions are free to move and carry a current
Are ionic substances (includes giant ionic lattice) brittle?
Yes, If enough force is applied, layers of ions slide over each other. This causes ions with the same charge to be brought next to each other. The strong repulsion between the same charges causes the ionic lattice to break apart.
What is a lone pair?
Pair of electrons which isn’t bonded
How is a double bond formed?
When 2 pairs of electrons (2 electrons from each atom) is shared
How is a triple bond formed?
When 3 pairs of electrons (3 electrons from each atom) is shared
What are the 2 notable exceptions of compounds that seem ionic but are actually covalent?
Beryllium chloride (BeCl2)
Aluminium chloride (AlCl3)
Explain co-ordinate (dative covalent) bonding.
Atom that donates electron has lone pair
Atom that accepts electron pair doesn’t have full outer shell of electrons, being electron deficient
This can be shown using an arrow, arrow points from atom donating the electrons e.g. (NH4)+ has N → H bond
Once formed it behaves like a regular covalent bond
What is the majority of covalent substances’ structure? and list the exceptions.
Simple molecular. The main exceptions are diamond, graphite, graphene and silicon dioxide. These covalent substances have a macromolecular structure (Giant covalent)
What is the physical properties of simple molecules (simple molecular)?
Exist as single molecules e.g. diatomic molecules
Low melting points
Generally insoluble
Does not conduct electricity
Give the simple molecular structure of iodine

Why do simple molecules have a low melting point?
Weak forces between molecules (Van der Waals), are overcomed when the solid is melted
What is the solubility of simple molecules?
Insoluble in H2O, soluble in oil (“like dissolves like” - a solute with Van Der Waals forces will dissolve in a solvent with Van der Waals forces)
Why do simple molecules not conduct electricity
No free moving charged particles to carry current
What is the shape of a molecule determined by?
Total number of electron pairs around central atom
Number of bonding pairs of electrons
Number of lone pairs of electrons
What is the Valence shell electron pair repulsion theory?
Pairs of electrons repel each other so that they are as far apart as possible
Lone pairs repel slightly more than bonded pairs as they are more compact
Molecule or ion takes up a shape which minimises these repulsions
Each lone pair reduces the bond angle by 2.5o
Memorise this
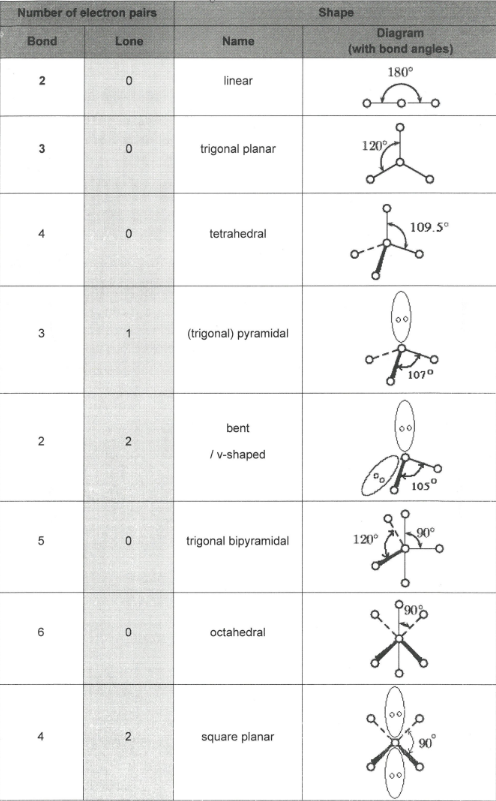
Explain why methane has a bond angle of 109.5o
C has 4 bond pairs of electrons which repel equally and spread themselves as far apart as possible, forming a tetrahedral shape
Explain why PCl3 has a bond angle of 107o
P has 3 bond pairs of electrons and 1 lone pairs of electrons. Lone pairs repel more than bond pairs. The shape is trigonal pyramidal
Explain why the C-O-H bond angle in methanol has a bond angle of 104.5o.
O has 2 bond pairs and 2 lone pairs of electrons. Lone pairs repel more than bond pairs. The shape around the O atom is bent
What happens to electronegativity across a period?
Increases because shared electron pair (in the covalent bond) is attracted more strongly due to the increasing number of protons in the nucleus of the atom
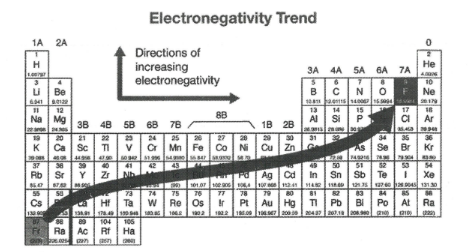
What happens to electronegativity down a group?
Decreases because the shared electron pair (in the covalent bond) is less strongly attracted due to the increased shielding from the nucleus of the atom
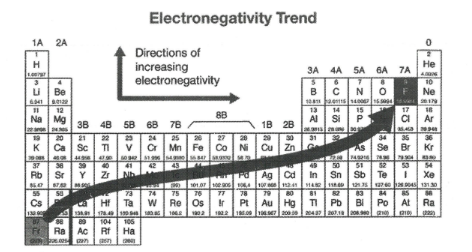
What is the most electronegative atom?
Fluorine
Why do noble gases not have electronegativity values?
Because they don’t normally form covalent bonds
How does the polarity of bonds work?
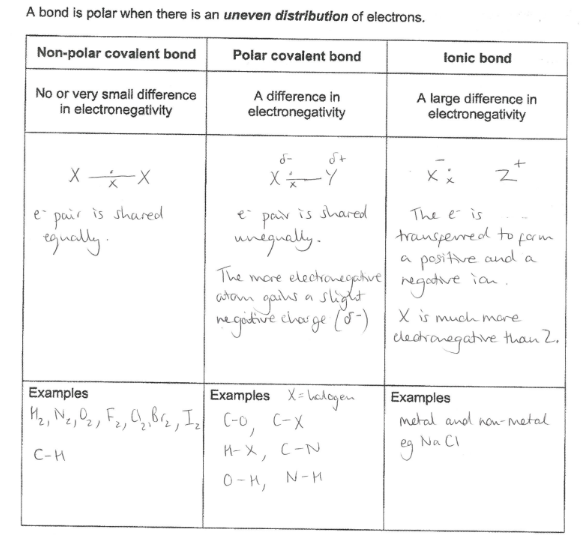
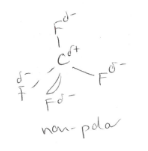
What is the polarity of CO2?

What is the polarity of H2O?

What is the polarity of CF4?
What are intermolecular forces? and what are the types?
forces of attraction between molecules, there are 3 types:
Van der Waals forces - weakest and present between all molecules
Permanent dipole-dipole forces - moderate in strength, present between polar molecules
Hydrogen bonds - strongest force between molecules which contain H-N, H-O, H-F
How does van der waals forces work?
Caused by movement of electrons which unbalances the charge distribution within the molecule, creating an instantaneous dipole across the molecule (which constantly forms and disappears). This in turn induces a dipole in neighbouring molecules, resulting in weak forces of attraction between molecules
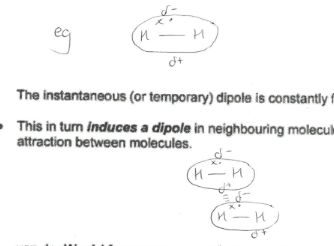
Which is van der waals the ONLY force present within a molecule?
non polar molecules, these substance have relatively low boiling points. They are generally gases or volatile liquids at room temperature.
What does the strength of van der waals forces depend on?
Size of molecule. Bigger molecules (greater Mr) has more electrons so induced dipoles are larger, resulting in stronger van der waals forces between molecules (large surface area also causes a stronger force)
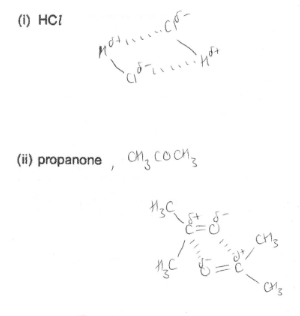
How does Permanent Dipole-Dipole Forces work?
Occur between molecules which have a permanent dipole. They occur in addition to van der waals forces. The δ+ end of one molecule is attracted to δ- end of a neighbouring molecule. Usually stronger than van der waals forces as a more permanent attraction
Give examples of permanent dipole-dipole forces.
What is the boiling point of substances with permanent dipole-dipole forces compared to van der waals forces?
Generally high boiling points than those with only van der waals forces and a similar Mr
Which has a high melting point I2 or HCl?
I2 has a high melting point than HCl even though HCl has stronger dipole-dipole forces between its molecules. This is because I2 has many more van der waals forces, which overall require more energy to overcome
What are compounds with dipole-dipole forces usually soluble in?
Polar solvents
How does hydrogen bonding work?
Occurs between molecules which contains (δ+)H bonded to a lone pair of electrons on either F,O or N in a neighbouring molecule (3 most electronegative atoms). They occur in addition to van der waals forces and is the strongest intermolecular force
Give examples of hydrogen bonding.

What are the properties of compounds with hydrogen bonding?
Higher boiling points than expected due to strength of hydrogen bonds between molecules
Tend to be soluble in water as they can form hydrogen bond with water molecules
Boiling point depends on number of hydrogen bonds formed per molecule
Hydrogen bonding explains two anomalous properties of water. (boiling point + density of solid (ice) ) . Pick and explain the graph
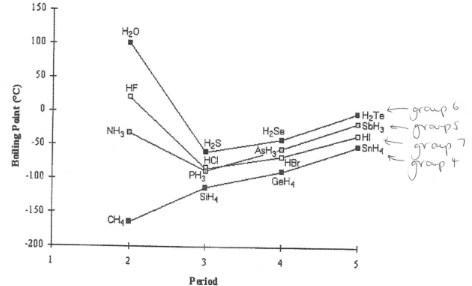
Trend as you go down groups (from period 2 to 5) is an increase in boiling point due to an increase in van der waals forces between molecules. NH3, HF and H2O do not fit trend as they also have much stronger H bonds between their molecules
What is the bonding of ammonium chloride?
Covalent, Dative covalent and ionic
(exam) Explain how lone pairs of electrons on oxygen influence bond angle in oxygen difluoride (OF2) (2)
Lone pairs repel more than bond pairs
Each lone pair reduces bond angle by approximately 2.5o
(exam) Deduce the type of intermolecular forces in SiF4. Explain how this type of intermolecular force arises and why no other type of intermolecular force exists in a sample of SiF4. (3)
Van der waals forces
Movement of electrons in one SiF4 molecule creates a temporary dipole. This induces a dipole in a nearby SiF4 molecule
There are no permanent dipole-dipole forces as the molecule is symmetrical and so dipoles cancel out
There is no hydrogen bonded to O or N or F, so no hydrogen bond
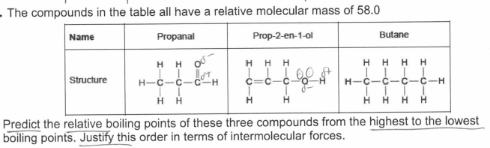
(exam) (6)
Butane has a boiling point lower than propanal which has a boiling point lower than prop-2-en-1-ol
Propanal has dipole-dipole forces between its molecules
Prop-2-en-1-ol has hydrogen bonds between its molecules
Butane only has van der waals forces between its molecules
Van der waals forces are weakest. Dipole-dipole forces are weaker than H bonds

(exam) (6)
I2 and HI are molecular
I2 and HI have covalent bonds between their atoms
I2 only has van der waals forces between its molecules.
HI has van der waals and dipole-dipole forces between its molecules
Dipole-dipole forces are stronger than van der waals forces.
However, I2 has a much larger Mr than HI and so has many more van der waals forces which require more energy to overcome
(exam) explain how permanent dipole-dipole forces arise between hydrogen chloride molecules (2)
Cl is more electronegative than H and so bond is polar
Cl(δ-) is attracted to H(δ+) on another HCl molecule