9 Kaplan MCAT Biochemistry Chapter 9: Carbohydrate Metabolism I
1/84
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
85 Terms
Metabolism
A set of chemical reactions through which an organism builds up or breaks down materials as it carries out its life processes
Anabolism
Metabolic pathways that construct molecules, requiring energy.
Catabolism
Metabolic pathways that break down molecules, releasing energy.

Glucose transport
Glucose enters cells by facilitated diffusion, transporters are called GLUT isoforms:
1) GLUT 1
2) GLUT 2
3) GLUT 3
4) GLUT 4
GLUT 2
Found in the liver (for glucose storage) and pancreatic Beta-islet cells.
A low-affinity glucose transporter found in the liver hepatocytes (for glucose transport) and pancreatic β-islet cells (as part of the glucose sensor for insulin release with glucokinase)
has a high Km (so low binding affinity) so the liver will pick up excess glucose and store it only after a meal, captures excess glucose primarily for storage
when glucose concentration drops below Km for the transporter, remainder bypasses liver and enters peripheral circulation

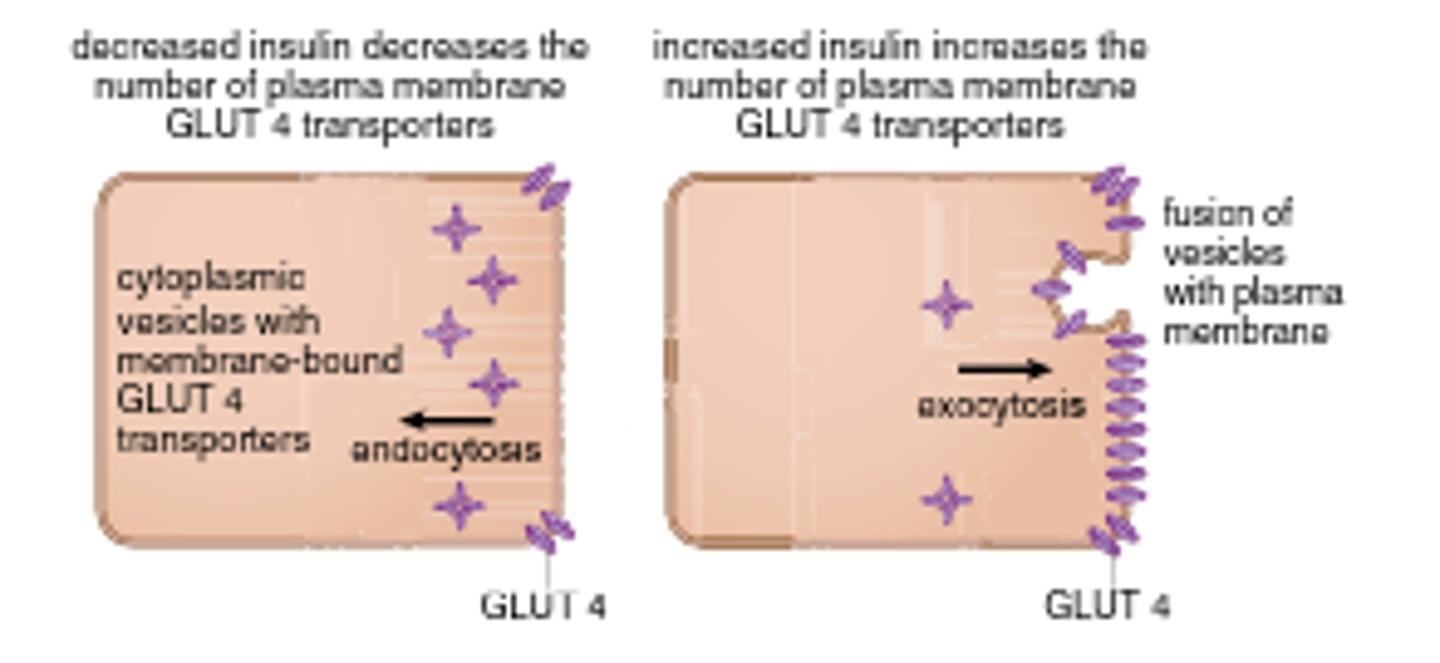
GLUT 4
Found in adipose tissue and muscles and is stimulated by insulin.
It has a low Km (so high binding affinity)
When stimulated by insulin, these cells will exocytose additional GLUT 4 enzymes into the PM to increase uptake and lower [blood glucose].
when person has high blood sugar concentrations, transporter will still permit only a constant rate of glucose influx because saturated (zero-order kinetics)
![<p>Found in adipose tissue and muscles and is stimulated by insulin.</p><p>It has a low Km (so high binding affinity)</p><p>When stimulated by insulin, these cells will exocytose additional GLUT 4 enzymes into the PM to increase uptake and lower [blood glucose].</p><p>when person has high blood sugar concentrations, transporter will still permit only a constant rate of glucose influx because saturated (zero-order kinetics)</p>](https://knowt-user-attachments.s3.amazonaws.com/d914c94e-d0fe-49b7-8557-d290b5aa4155.jpg)
how can cells with GLUT 4 transporters increase their intake of glucose?
increase number of GLUT4 transporters on their surface
Insulin's Action:
Insulin binds to receptors on the cell surface, triggering the PI3K/Akt signaling pathway. This pathway then regulates GLUT4 trafficking, moving GLUT4 from intracellular storage vesicles (GSVs) to the plasma membrane (PM).
Type I and II diabetes
Diabetes mellitus is caused by a disruption of insulin/GLUT 4 mechanism. In type I diabetes, insulin is absent and cannot stimulate the insulin receptor (therefore, no additional GLUT 4 added to PM to reduce [blood glucose]).
In Type II, the receptor becomes insensitive to insulin and no GLUT 4 are added to PM.
Both result in high levels of blood glucose.
Inflammation of beta cells, or "insulitis," is a key feature of type 1 diabetes where the immune system mistakenly attacks and destroys these insulin-producing cells, leading to insulin deficiency.
A major defect in insulin resistance is the inability of insulin to effectively signal the translocation of GLUT4 from intracellular storage vesicles to the cell surface.
![<p>Diabetes mellitus is caused by a disruption of insulin/GLUT 4 mechanism. In type I diabetes, insulin is absent and cannot stimulate the insulin receptor (therefore, no additional GLUT 4 added to PM to reduce [blood glucose]).</p><p>In Type II, the receptor becomes insensitive to insulin and no GLUT 4 are added to PM.</p><p>Both result in high levels of blood glucose.</p><p>Inflammation of beta cells, or "insulitis," is a key feature of type 1 diabetes where the immune system mistakenly attacks and destroys these insulin-producing cells, leading to insulin deficiency.</p><p>A major defect in insulin resistance is the inability of insulin to effectively signal the translocation of GLUT4 from intracellular storage vesicles to the cell surface.</p>](https://knowt-user-attachments.s3.amazonaws.com/2825b40c-f2fb-4be5-83cd-dad36fdcc084.jpg)
GLUT 4 Insulin picture
adipose tissue requires glucose to form
dihydroxyacetone (DHAP) which is converted to glycerol phosphate to store incoming fatty acids as triacylglycerols
Glucose Metabolism
Glycolysis: the breakdown of glucose to pyruvate to yield energy.
Gluconeogenesis: The combination of small carbon containing compounds to yield glucose.
Glycogen synthesis and breakdown: the storage and recovery of glucose from glycogen polymer.
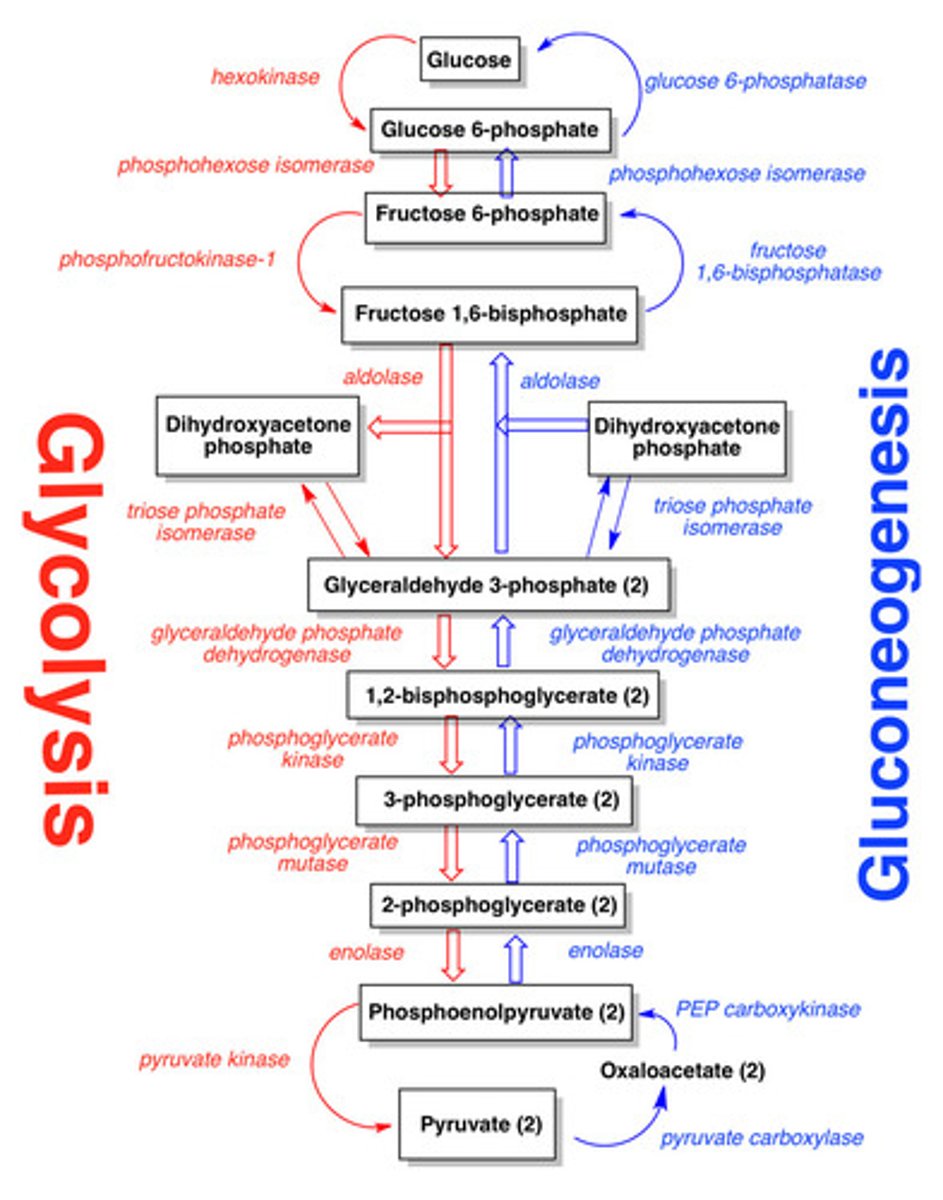
Glycolysis Overview
(Draw pathway)
Glycolysis occurs in a total of 10 steps:
Glucose is broken down into two pyruvate (3-carbon molecules)
The first 5 steps are the energy investment phase (2 ATP molecules hydrolyzed to ADP per glucose)
The second step (6-10) are the energy payoff phase.
4 ATP molecules are generated per glucose for a net gain of 2 ATP.
2 NAD+ election carriers are reduced to 2 NADH
NADH can be subsequently oxidized to generate additional ATP in the ETC.
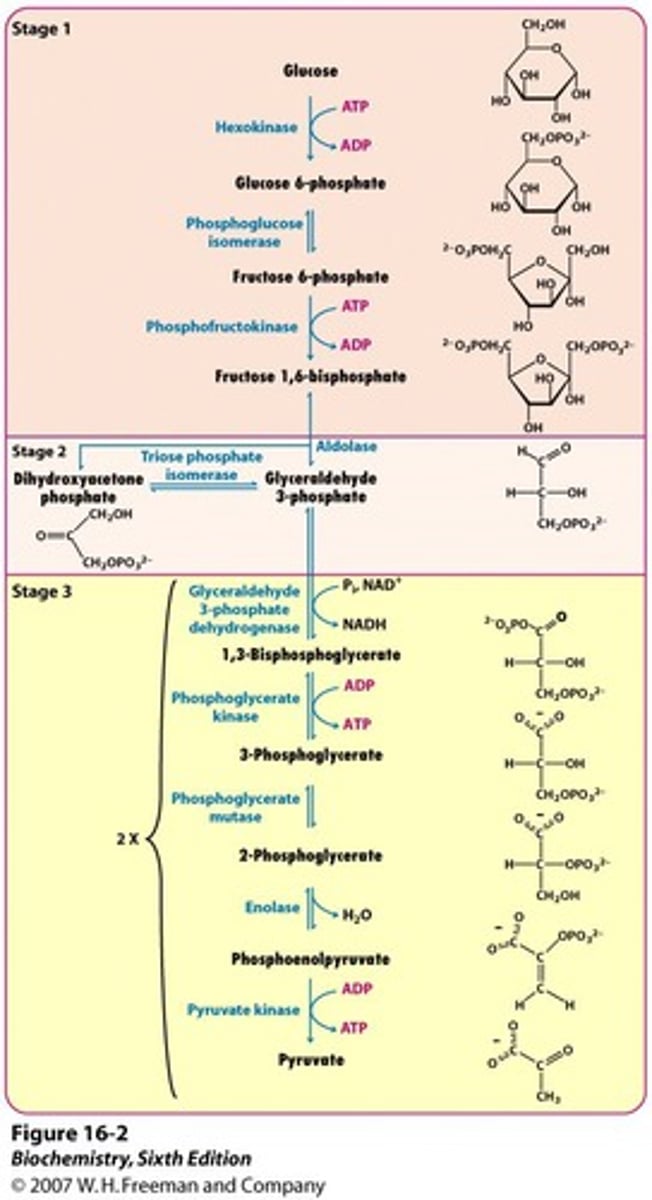
Important Enzymes of Glycolsis
hexokimasenase
Phosphofructo kinase
G3P dehydronase
Phosphoglecerate kinase
Pyruvate kinase
Energy Investment Phase
2 ATP -> ADP

Energy Payoff Phase
4 ADP -> 4 ATP

Glycolysis Step 1
glucose enters cell by facilitated diffusion or active transport and kinases convert glucose to glucose 6-phosphate, GLUT transporters are specific to glucose (not phosphorylated glucose), the glucose gets "trapped" in the cell and can't leak out
1 ATP -> 1 ADP

Explain different enzymes in Step 1:
1) Glucokinase - induced by insulin, converts glucose to glucose-6-phosphate. It is present in the pancreas (B-islet cells) and liver. It allows for increased uptake of glucose in liver for glycogen storage. high Km (acts on glucose proportionally to its concentration/ Doesnt get saturated easily
2) Hexokinase - inhibited by its product--> glucose 6-phosphate, converts glucose to glucose-6-phosphate in all other tissues. low km (reaches maximum velocity at low glucose concentration)
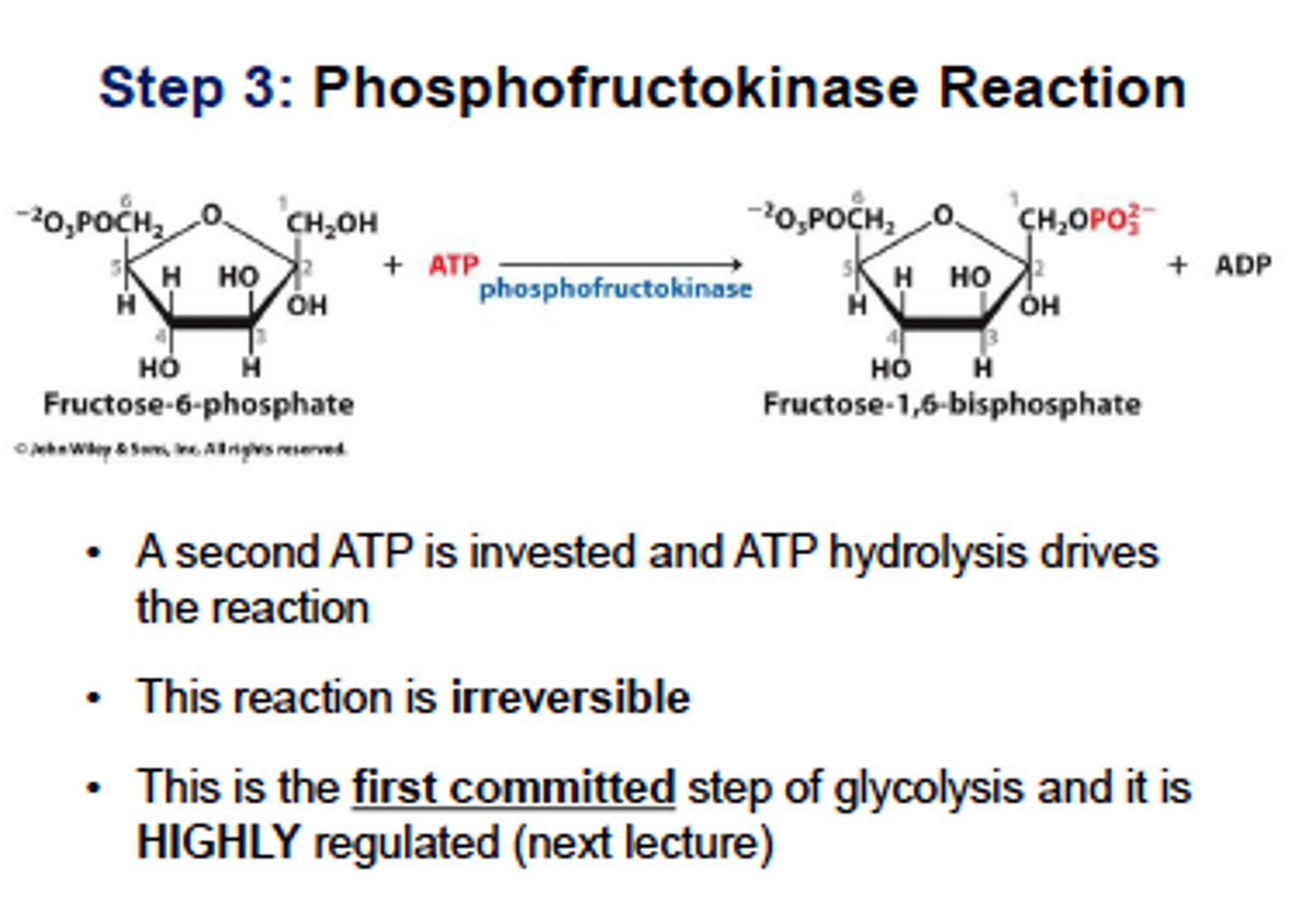
Glycolysis Step 3 (PFK1 and 2)
phosphofructokinase
fructose 6-phosphate is phosphorylated to fructose 1,6-biphosphate using ATP
PFK-1 is the rate-limiting enzyme and main control point in glycolysis.
PFK-1 is inhibited by ATP and citrate, and activated by AMP. Makes sense because the cell should turn off glycolysis when it has sufficient energy (high ATP) and turn it on when it needs energy (high AMP)
Insulin stimulates PFK-1, which allows cells to override inhibition caused by ATP so that glycolysis can continue even when the cell is energetically satisfied
Glucagon inhibits PFK-1 in hepatocytes by indirect mechanism involving PFK-2 and fructose 2,6- biphosphate
PFK-2- found mostly in liver, activated by insulin which converts tiny amount of fructose 6-phosphate to fructose 2,6-biphosphate which avtivates PFK-1.
glucagon inhibits PFK-2, lowering F2,6-BP and inhibiting PFK-1
metabolites of glycolysis can thus be fed into the production of glycogen, fatty acids, and other storage molecules rather than just being burned to produce ATP
1 ATP -> 1 ADP
Rate limiting steps of Carbohydrate metabolsim
Glycolosis: PFK-1
Fermentation: lactate dehydrogenase
glycogenesis: glycogen synthaseGly coogenolysis: glycogen phosphoorylase
gluconeogenesis: fructose- 1,6 bisphosphotase
PPP: glucose-g-phosphate dehydrogenase
Glyceraldehyde-3-phosphate dehydrogenase
Catalyzes an oxidation and addition of inorganic phosphate to its substrate, glyceraldehyde 3-phosphate, which results in the intermediate 1,3-bisphosphoglycerate and reduction on NAD+ to NADH
NADH can be oxidized if in aerobic glycolysis by electron transport chain, providing ATP synthesis

3-phosphoglycerate kinase
Transfers the high-energy phosphate from 1,3-bisphosphoglycerate to ADP, forming ATP and 3-phosphoglycerate
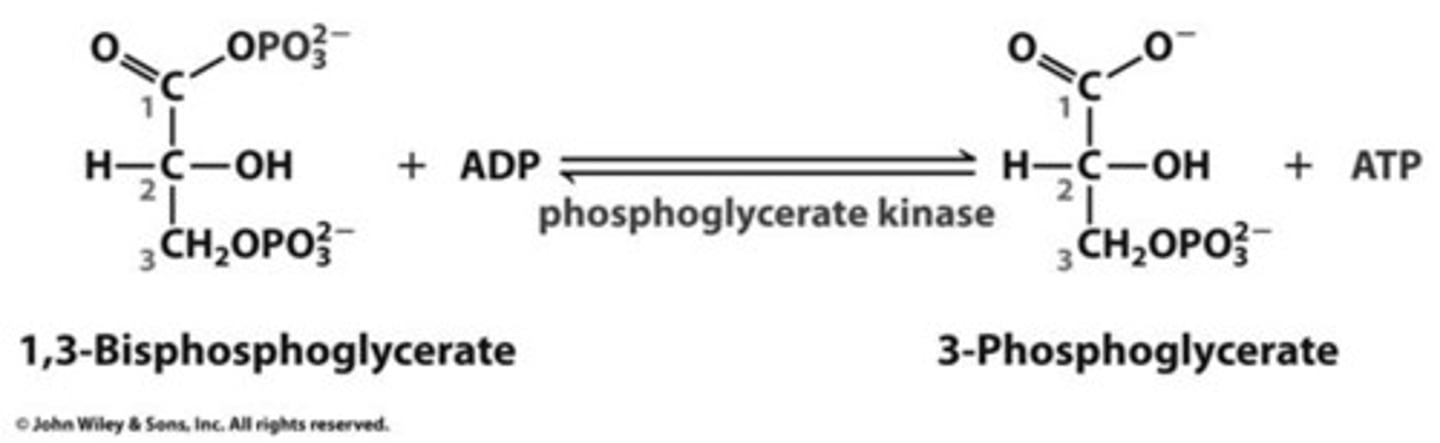
substrate-level phosphorylation
The enzyme-catalyzed formation of ATP by direct transfer of a phosphate group to ADP from an intermediate substrate in catabolism. not dependent on oxygen
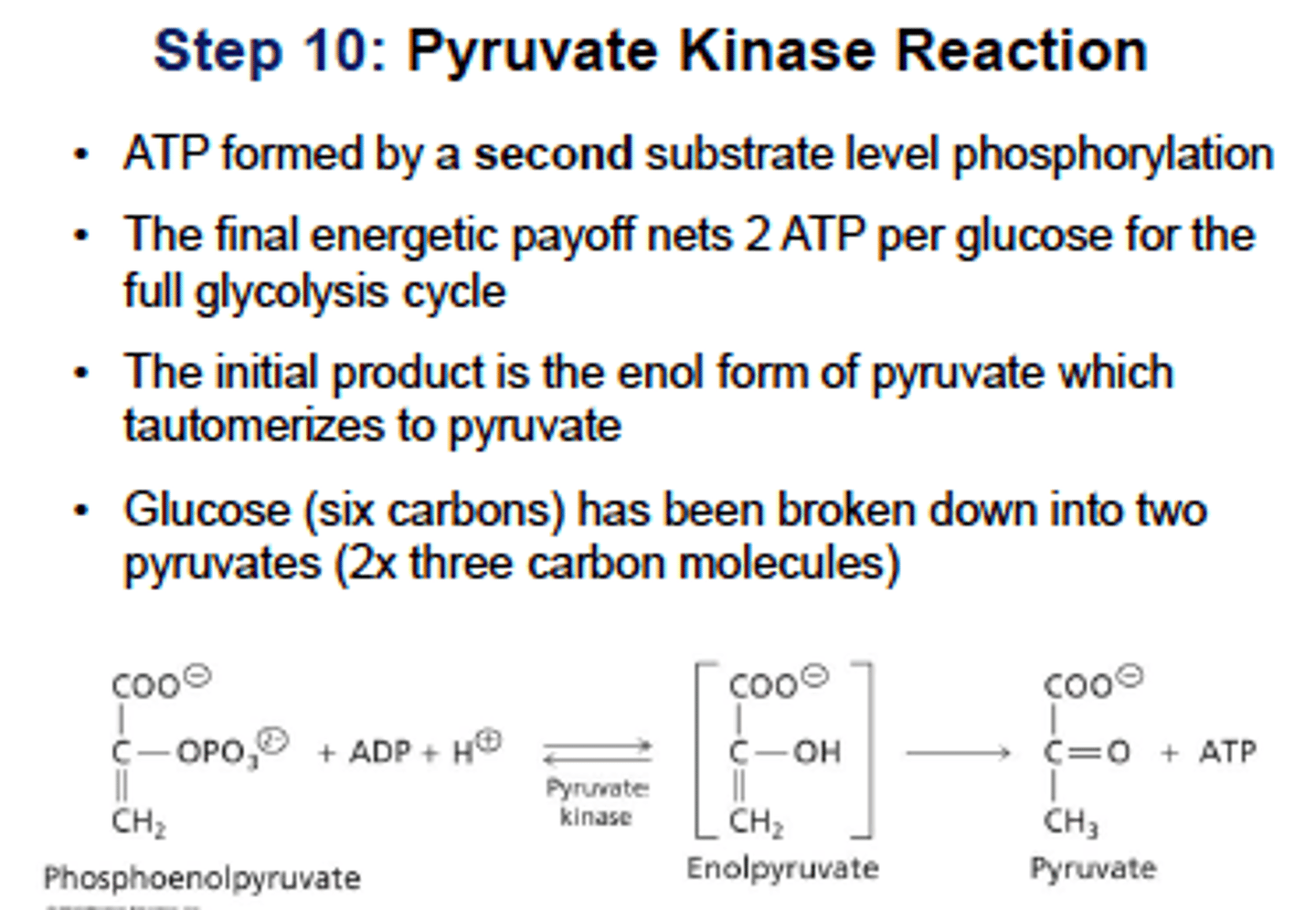
Glycolysis Step 10: (pyruvate kinase)
feed-forward activation - activated by fructose 1,6-bisphosphate from PFK-1 reaction

pyruvate kinase
Catalyzes substrate-level phosphorylation of ADP by PEP (phosphoenolpyruvate) to form pyruvate, stimulated in feed-forward activation by F-1,6-BP from PFK-1 reaction
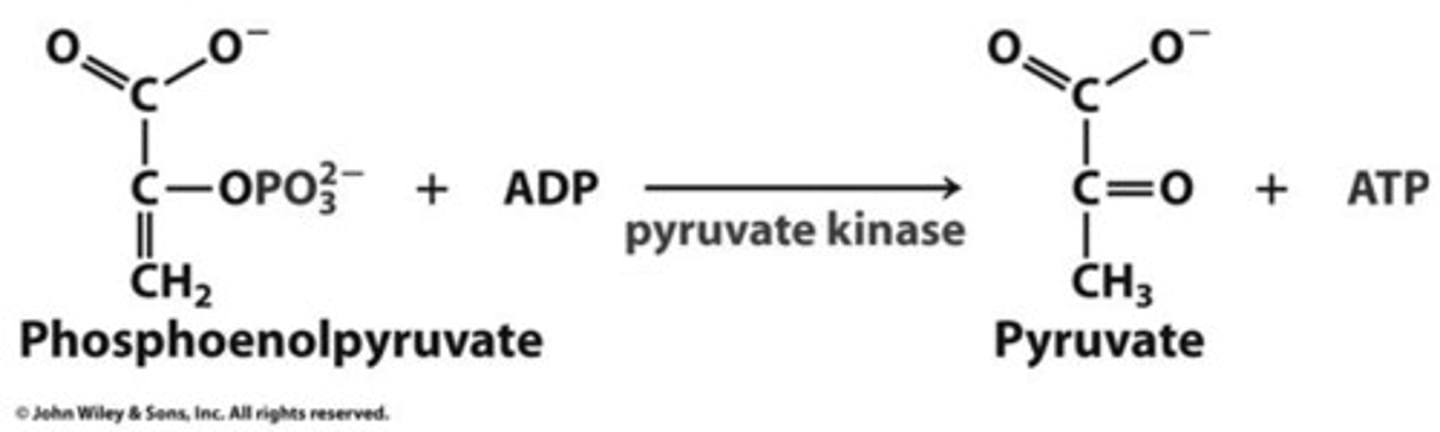
feed-forward reaction
product of an earlier reaction stimulates/ prepares a later reaction
ex. PFK 1 makes fructose 1,6 phosphate which stimulates pyruvate kinase (another enzyme) necessary to make pyruvate and finish glycolysis
ex. glucose 6 phosphate stimulates glycogen synthase in glycogenesis
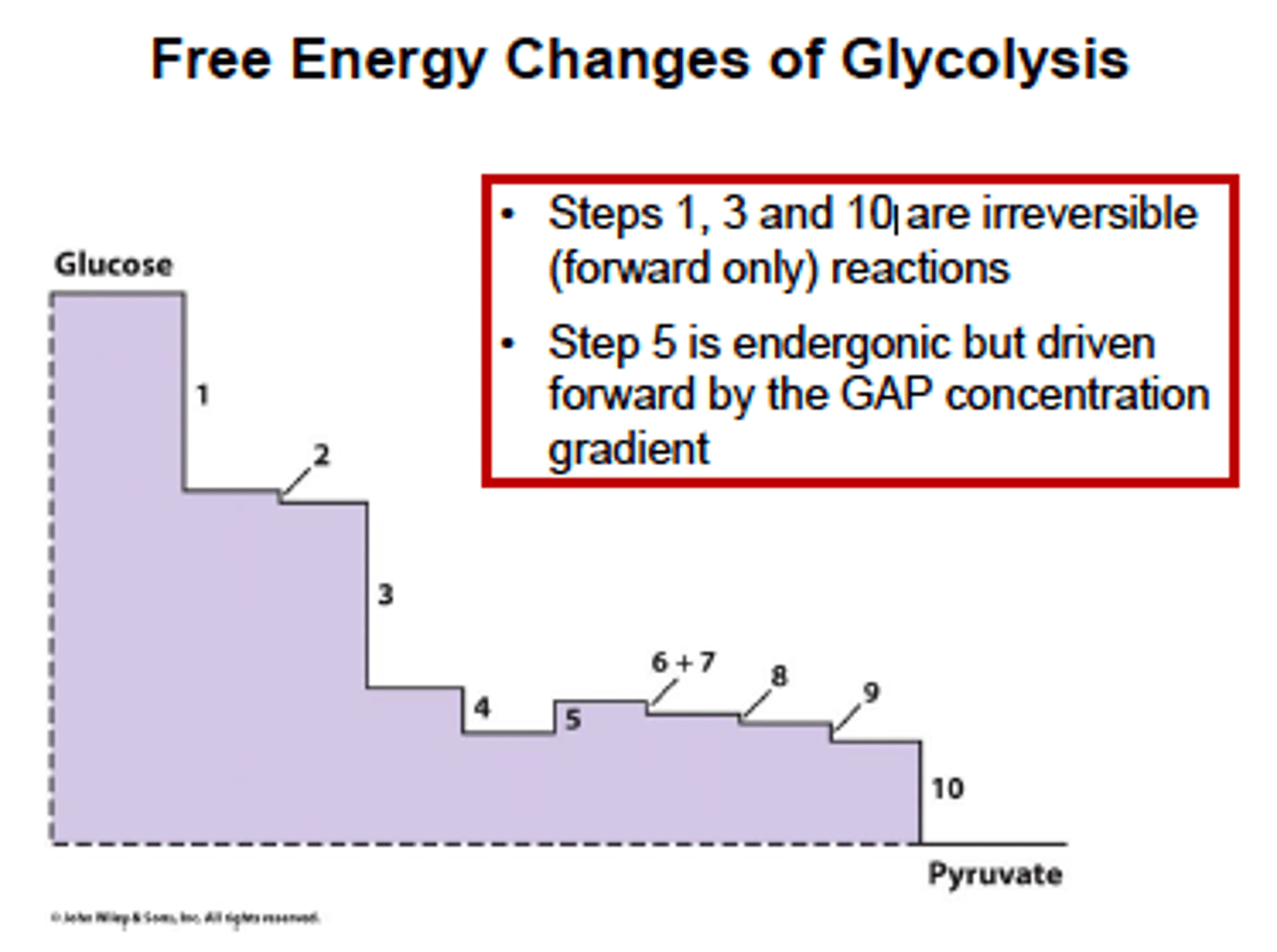
Free energy change in Glycolysis?
step 5 is glyceraldehyde-3-phosphate dehydrogenase
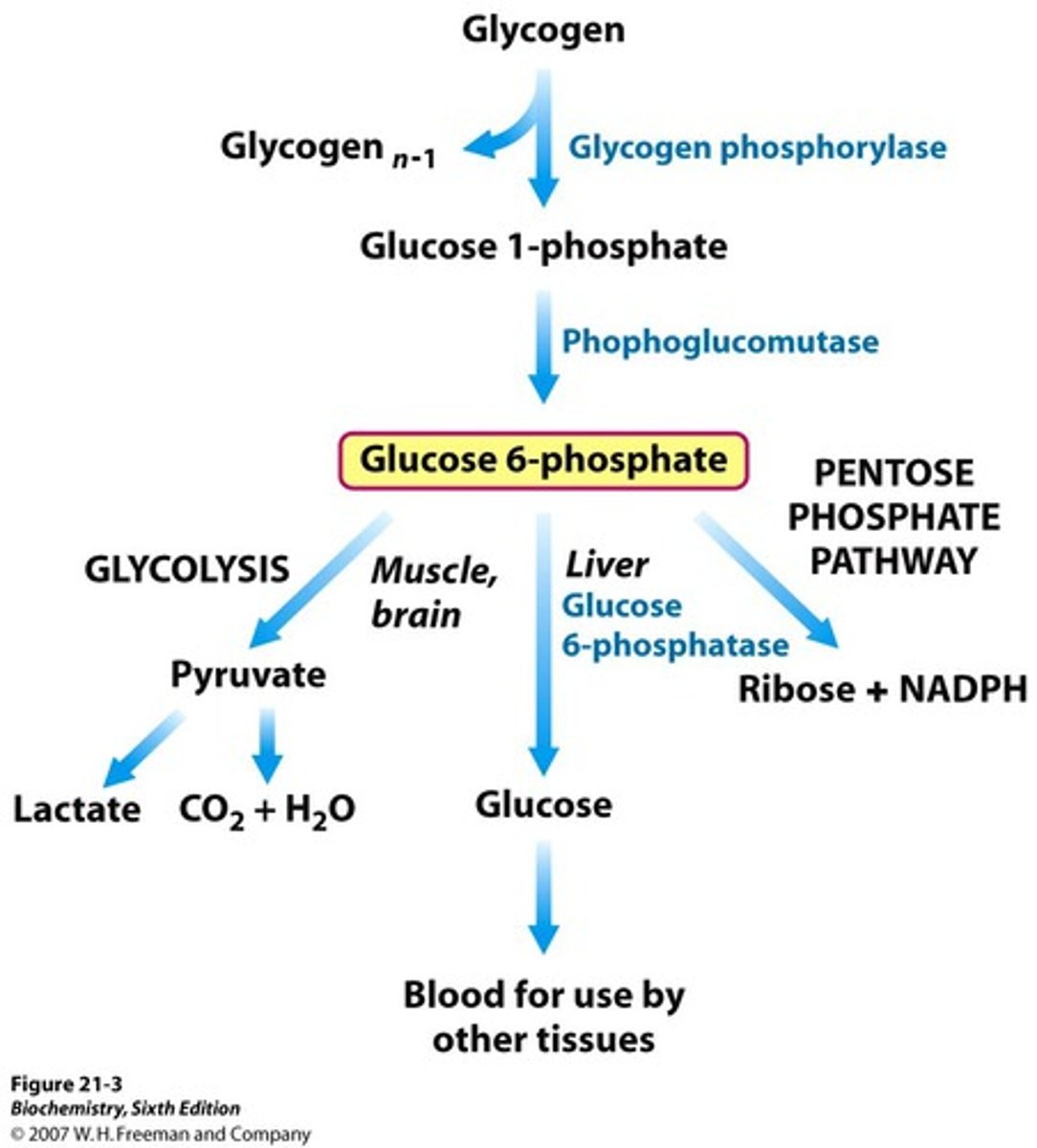
Metabolic pathways for Glucose-6-phosphate?

Anaerobic Glycolysis
(Draw pathway)
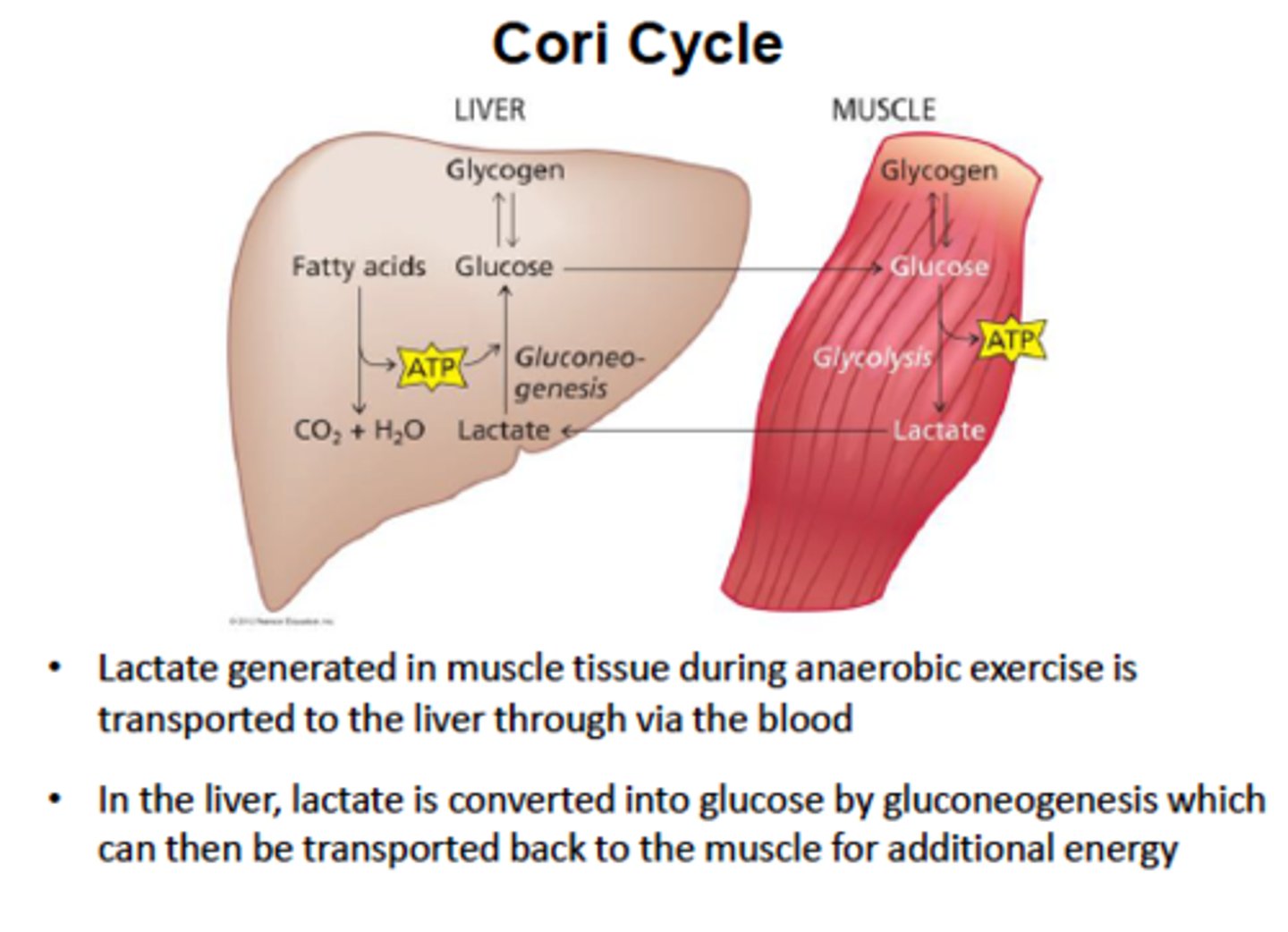
Occurs when either mitchondria or oxygen are lacking (like bloood cells oor exersing muscles)
Cori Cycle
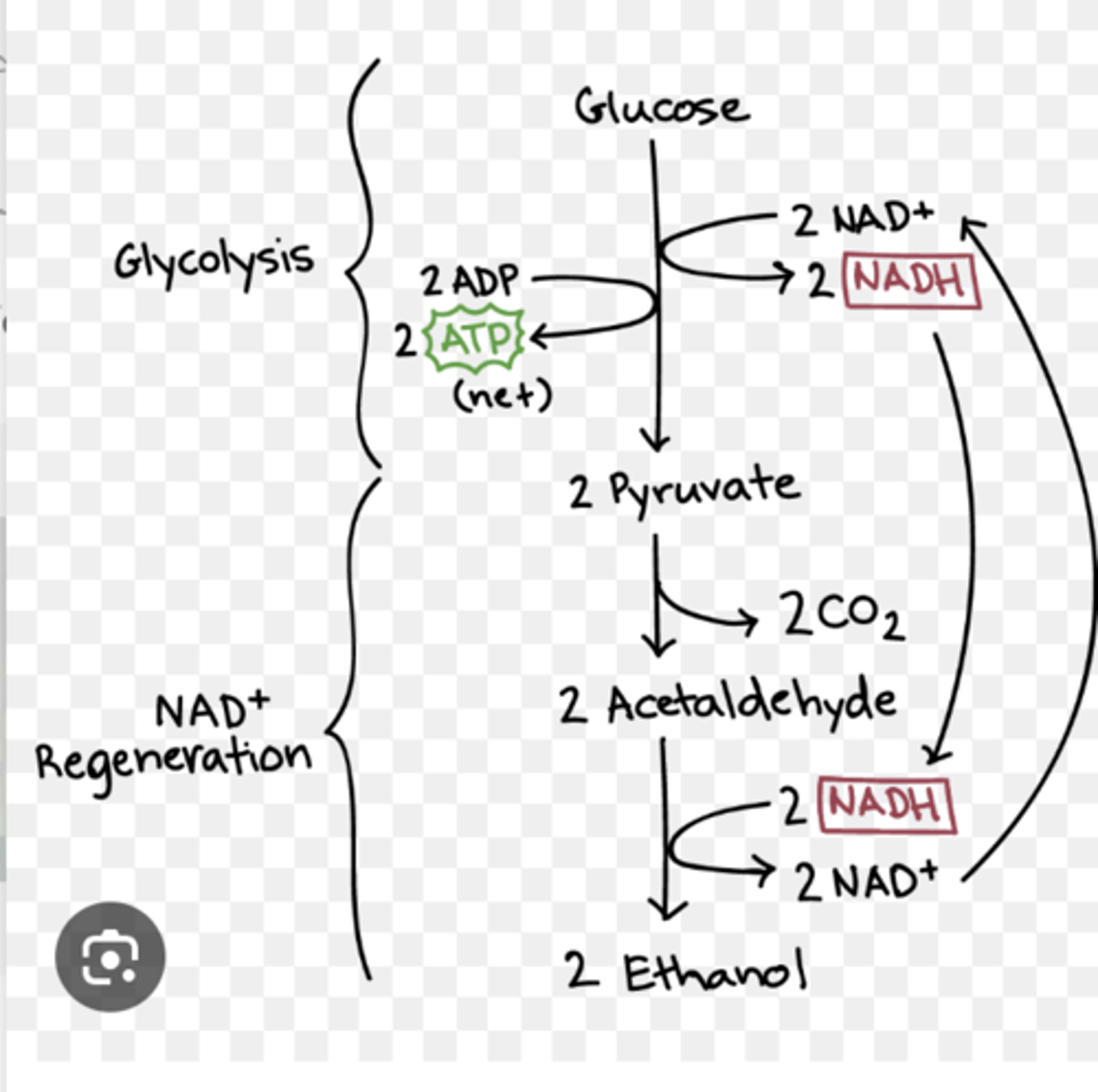
Fermentation
(Draw Pathway)
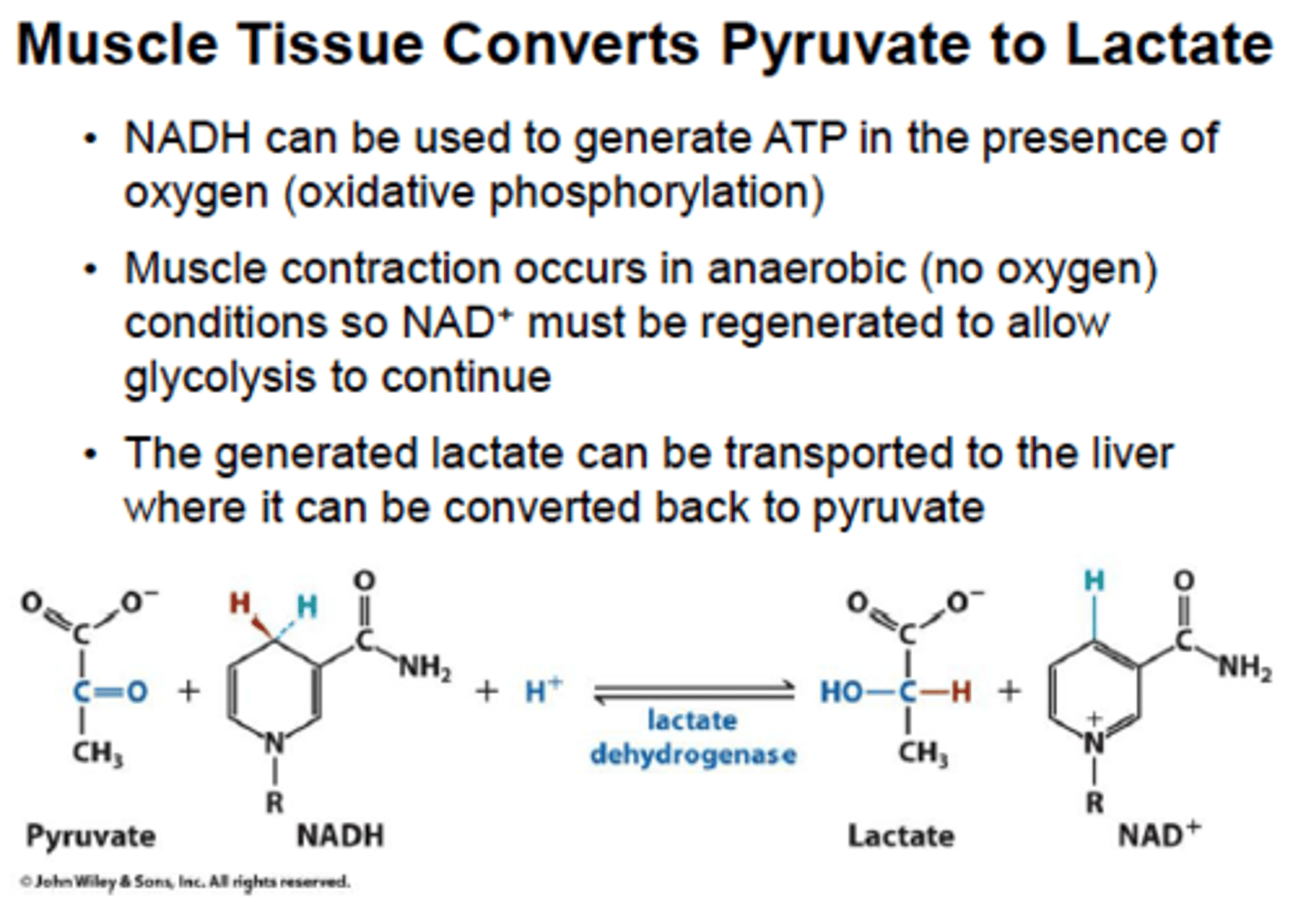
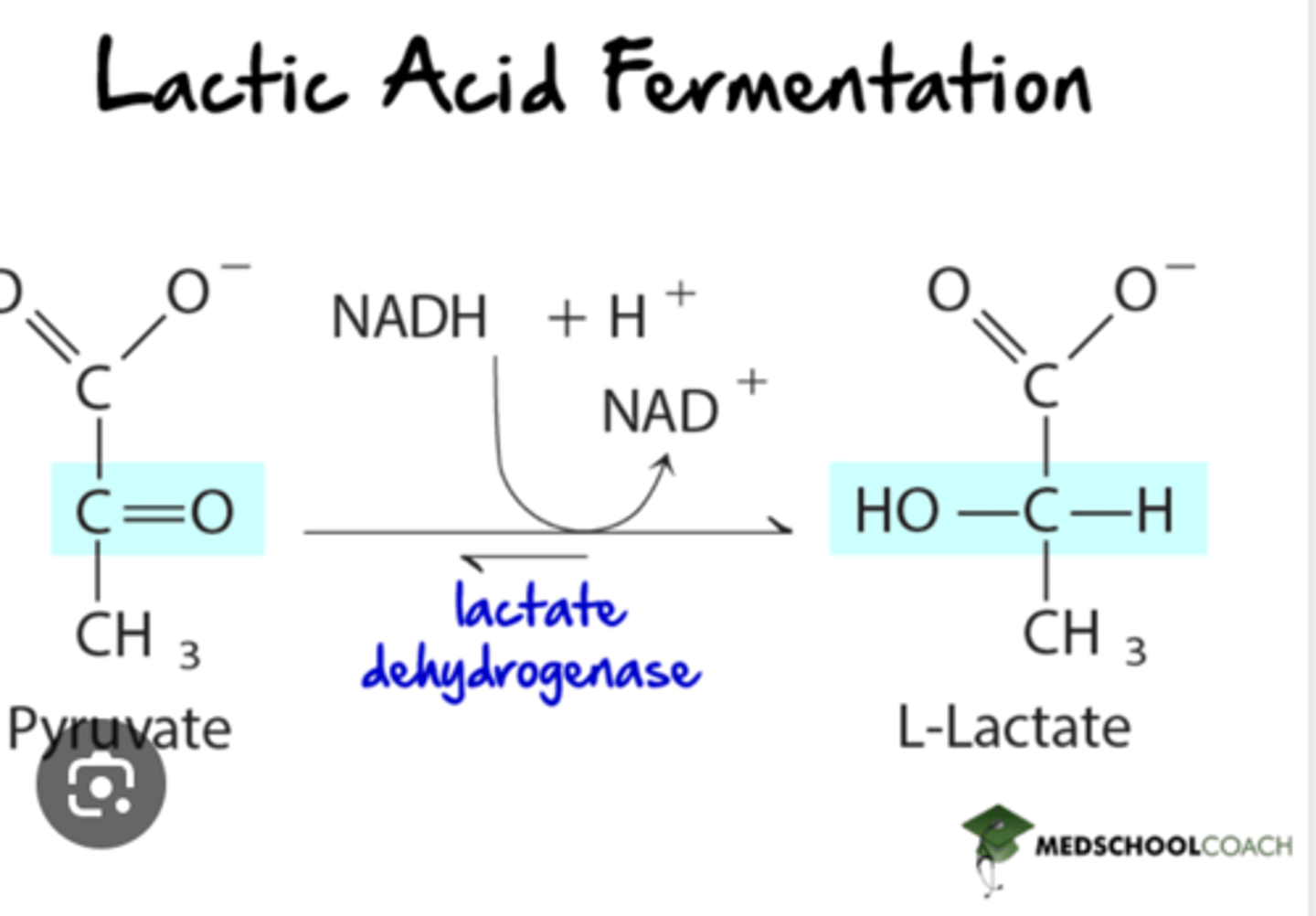
If oxygen or mitochondria are absent, the NADH produced in glycolysis is oxidized by cytoplasmic lactate dehydrogenase. (RBCs, skeletal muscle)
lactate dehydrogenase: NADH -> NAD+, which replenishes NAD+ so glycolysis can continue
by reducing pyruvate to lactate and oxidizing NADH to NAD+, lactate dehydrogenase prevents this potential problem for developing
Starch metabolism
The process o
fermentation (anaerobic respiration) in yeast
ethanol and carbon dioxide
Glycolysis Intermediates: DHAP
DHAP (Dihydroxyacetone phosphate) is used in haptic and adipose tissue for triacylglycerol synthesis. Formed from 1,6-biphosphate. Can be isomerize to glycerol 3-phosphate which can then be converted to glycerol, the backbone of triacylglycerols

1,3-BPG and PEP
biphosphoglycerate and phosphoenolpyruvate
High energy intermediates used to generate ATP by substrate-level phosphorylation. only ATP gained in anaerobic respiration
mnemonic for irreversible steps of glycolysis
How Glycolysis Pushes Forward the Process: Kinases
•H = hexokinase
•G = glucokinase
•PF = PFK-1
•PK = pyruvate kinase
Keeps the pathway moving in only one direction.
In gluconeogenesis, different rxn and enzymes must be used at these steps in the reverse pathway
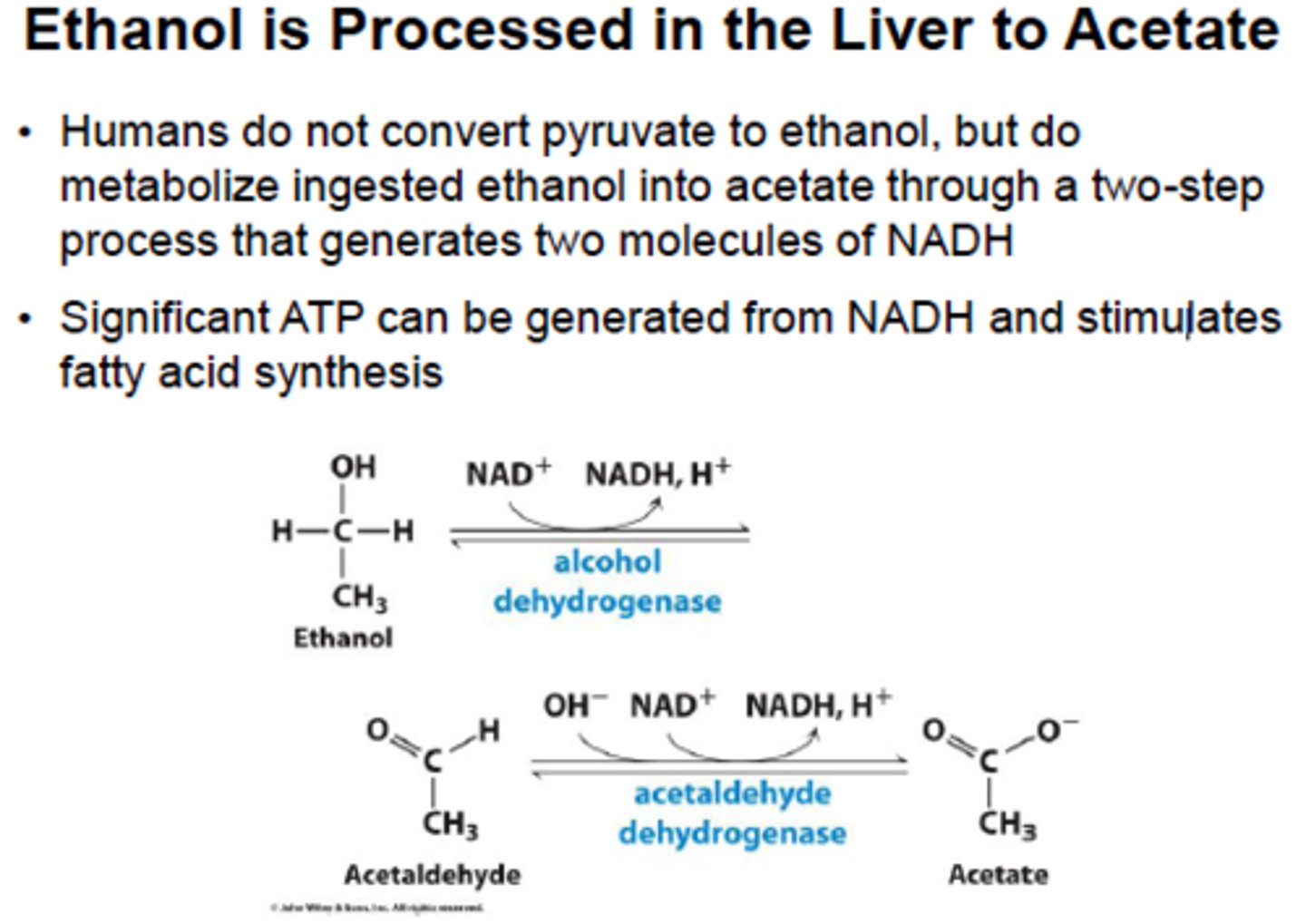
Ethanol metabolism:
(Draw pathway)
Metabolism in Erythrocytes
In red blood cells, glycolysis is the only pathway for ATP production, yielding 2 net ATP.
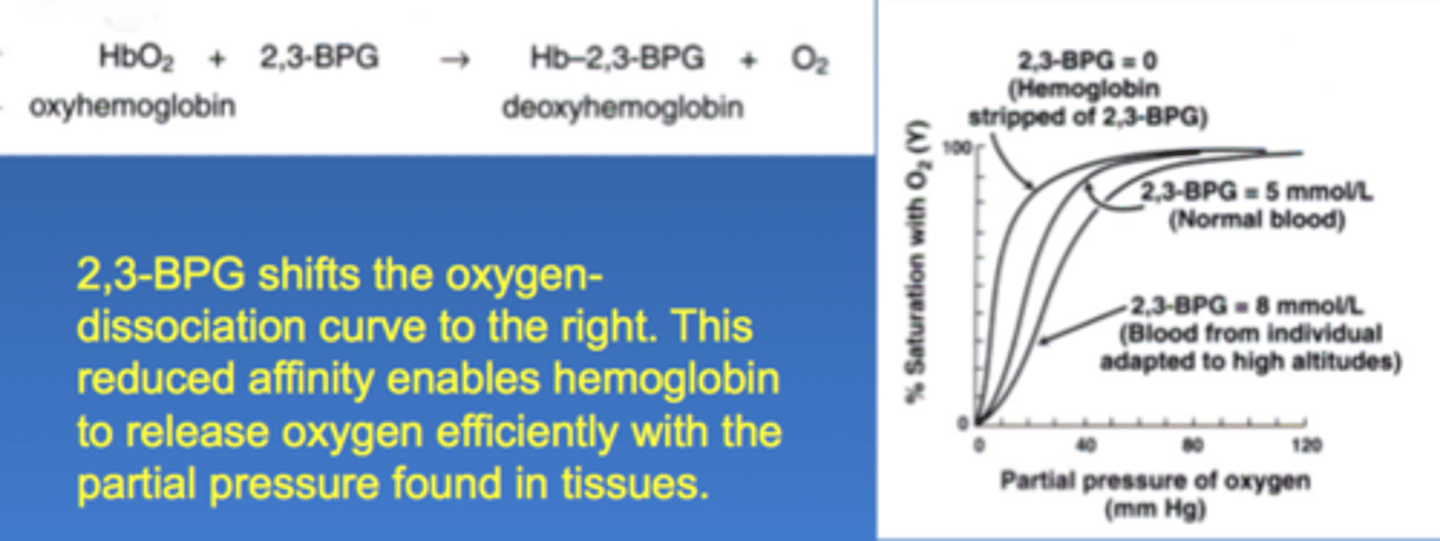
1) Contain bisphosphoglycerate mutase to convert 1,3-bisphosphoglycerate to 2,3-BPG.
2,3-BPG binds allosterically to the B-chains of hemoglobin A (HbA) and decrease affinity for oxygen
High 2,3 BPG causes a right shift, causing a release of oxygen
fetal hemoglobin
2,3-BPG does not bind well with result that HbF has higher affinity for oxygen than maternal HbA, allows transplacental passage of oxygen from mother to fetus
physiological change that promote a right shift of the oxygenn dissosiation curve
high 2,3-BPH
low pH
high pCO2
Glycolysis rate limiting step
PFK-1: Upregulated by fructose- 2,6 bisphosphate and AMP; Downregulated by ATP and Citrate
Other monosaccharides
fructose and galactose
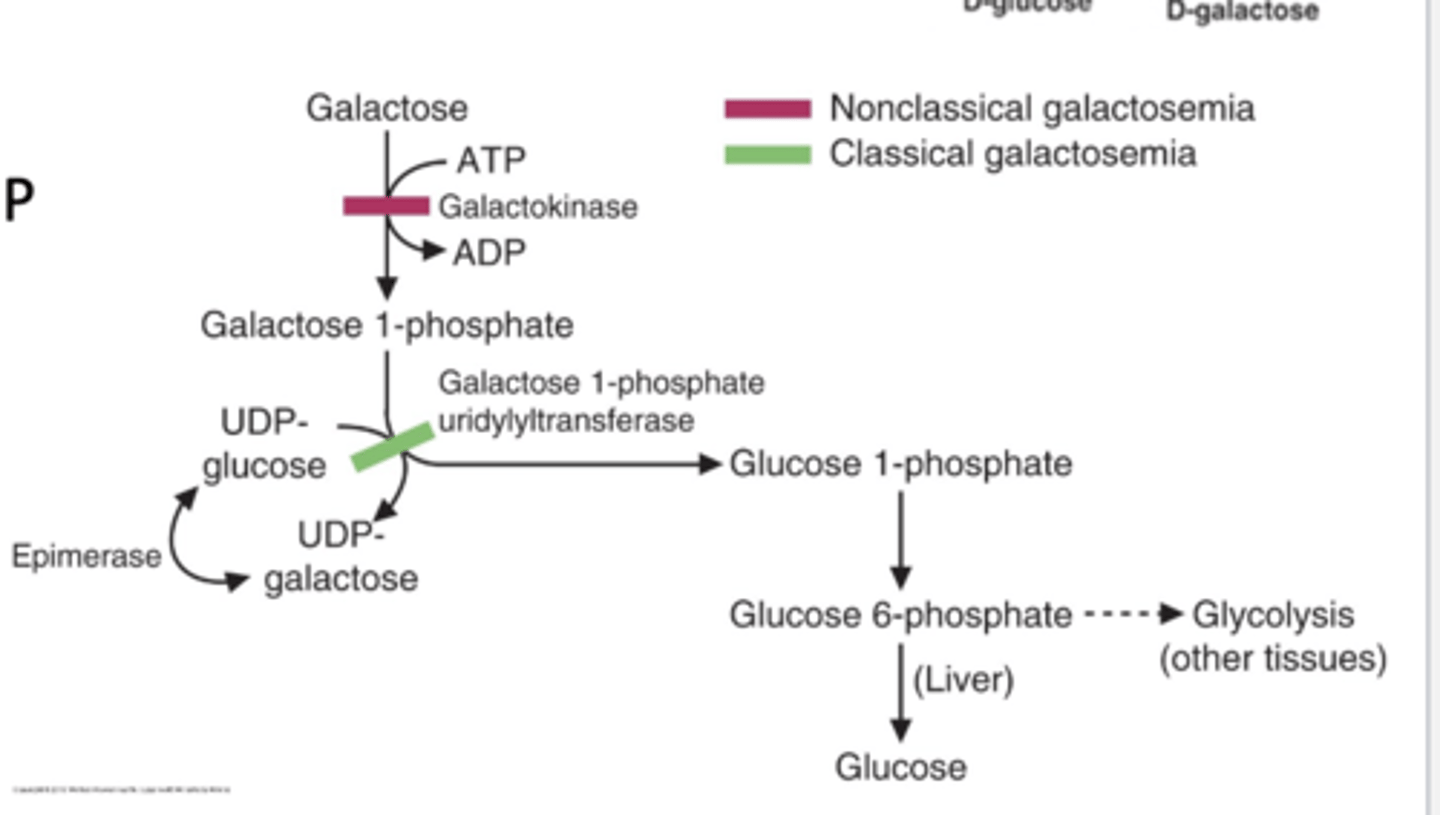
Galactose metabolsim
galcactose -> galctose-1- glucose 1-P -> glucose-6-P
then enzymes:
galactokinase-> gal-1-P uridyltransferase annd an epimerase
Pyruvate Dehydrogenase
inside the mitochondria
Complex of enzymes that convert pyruvate -> acetyl-CoA
stimulated by insulin
inhibited by acetyl-CoA
requires: thaimine pyrophosphate, lipoic acid, CoA, FAD, NAD+
irreversible
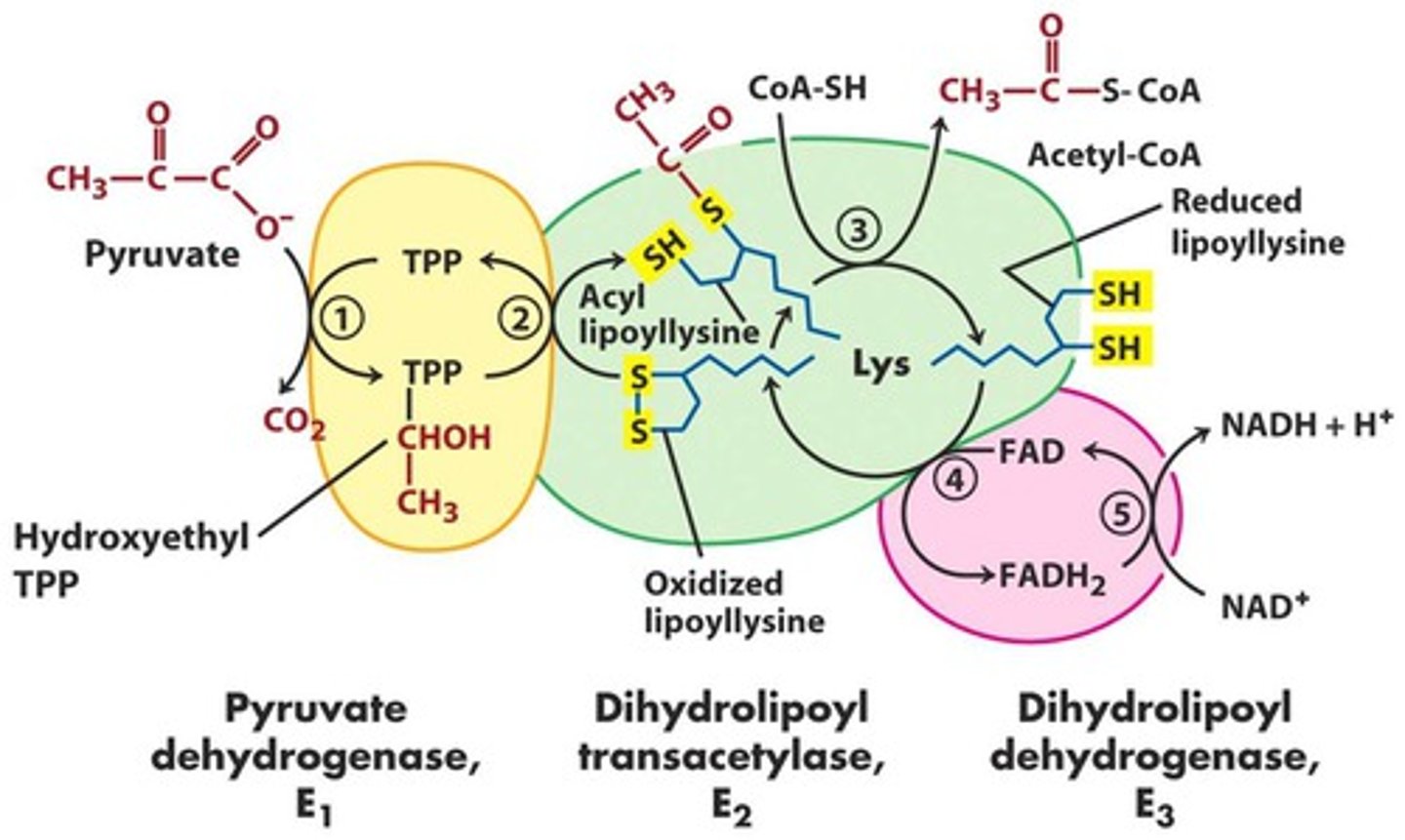
reactants and products of pyruvate dehydrogenase
reactants: pyruvate, NAD+, CoA
products: acetyl-CoA, NADH, CO2
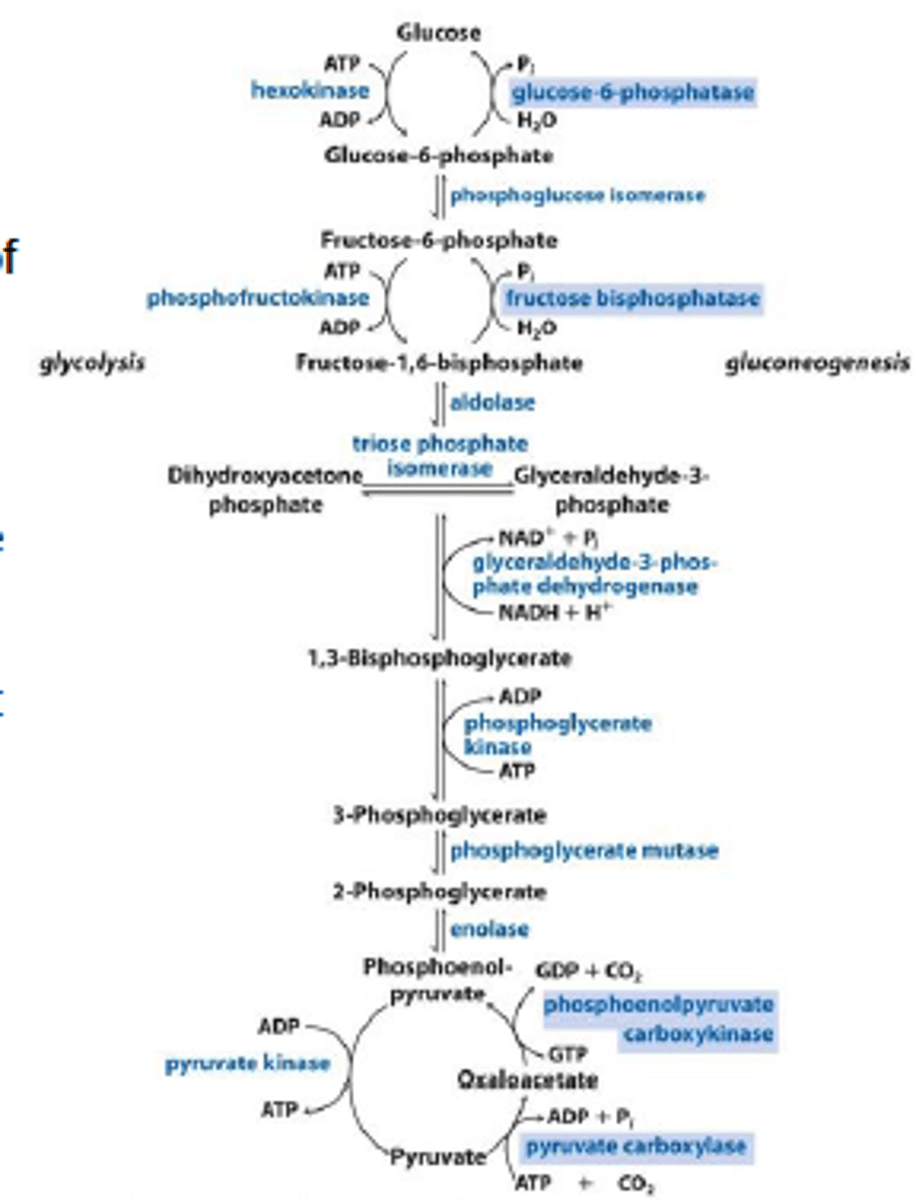
Gluconeogenesis
The formation of glucose from pyruvate (glycolysis) or oxaloacetate (TCA cycle).
Acetyl-CoA can't be converted back to glucose
glucogenic amino acids
all except leucine and lysine; can be converted into intermediates that feed into gluconeogenesis
ketogenic amino acids
can be converted into ketone bodies, which can be used as an alternative fuel, particularly during periods of prolonged starvation
important substrates for gluconeogenesis
glycerol 3-phosphate (from stored fats, or triacylglycerols in adipose tissue); lactate (from anaerobic glycolysis); glucogenic amino acid (from muscle proteins)
lactate is converted to pyruvate by
lactate dehydrogenase
alanine is converted to pyruvate by
alanine aminotransferase
glycerol 3-phophate is converted to DHAP by
glycerol-3-phosphate dehydrogenase
Fates of pyruvate
1. conversion to acetyl CoA by PDH
2. conversion to lactate by lactate dehydrogenase
3. conversion to oxaloacetate by pyruvate carboxylase
when there is a build up of acetyl CoA, this inhibits PDH and so the CoA goes to Beta Oxidation
also pyruvate is no longer converted into acetyl CoA but goes to make oxaloacetate
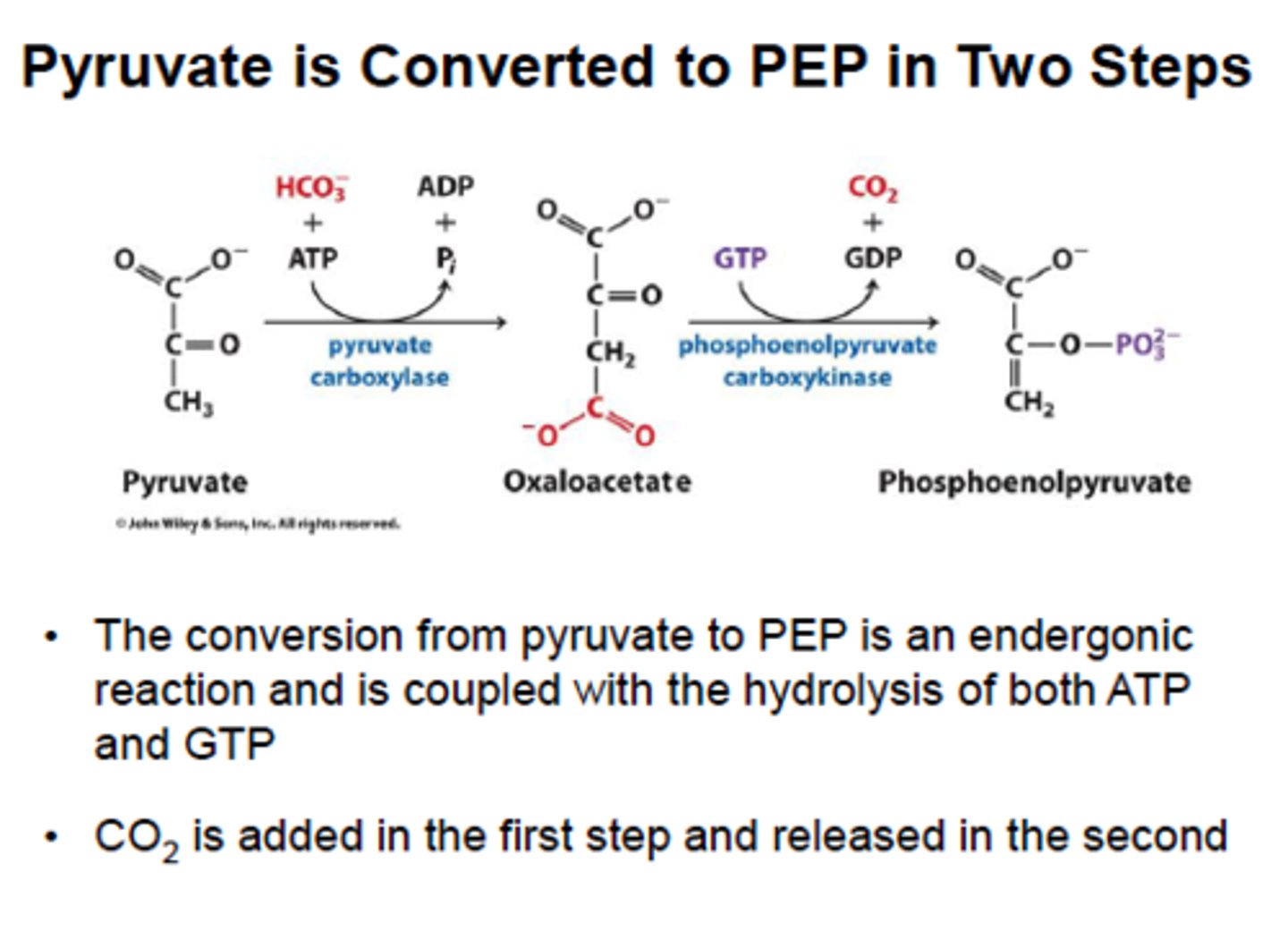
Gluconeogenesis Step 1: pyruvate carboxylase and PEPCK
Pyruvate carboxylase converts pyruvate into oxaloacetate, which is converted to phosphoenolpyruvate (PEP) by PEPCK. These 2 enzymes bypass pyruvate kinase in glycolysis.
pyruvate carboxylase is activated by acetyl-CoA from Beta-oxidation (FROM FATTY ACIDS) thus fatty acids must be burned in liver to provide energy, stop the forward flow of CAC and produce massive amounts of OAA that can eventually lead to glucose production for the rest of the body
PEPCK is activated by glucagon and cortisol
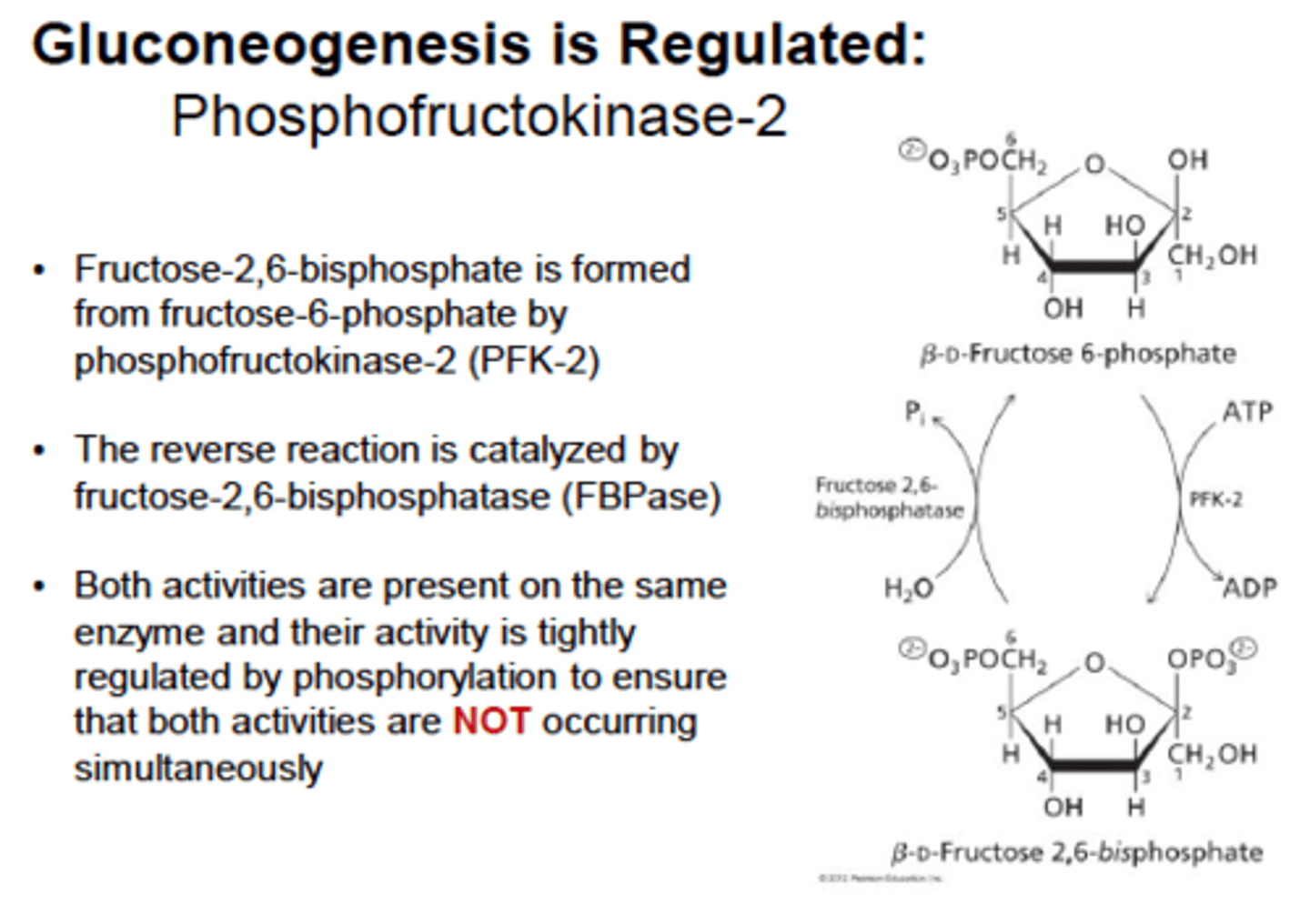
Gluconeogenesis 8: fructose-1,6-biphosphate
Fructose 1,6 Bisphosphate converts fructose 1,6-bisphosphate to fructose 6-phosphate, bypassing PFK-1. This is the rate-limiting step of gluconeogenesis
Fructose 1,6 Bisphosphate is activated by ATP directly and glucagon indirectly
Inhibited by AMP directly and insulin indirectly
F2,6-BP glucagon will lower it and stimulate gluconeogenesis whereas insulin will increase it and inhibit gluconeogenesis
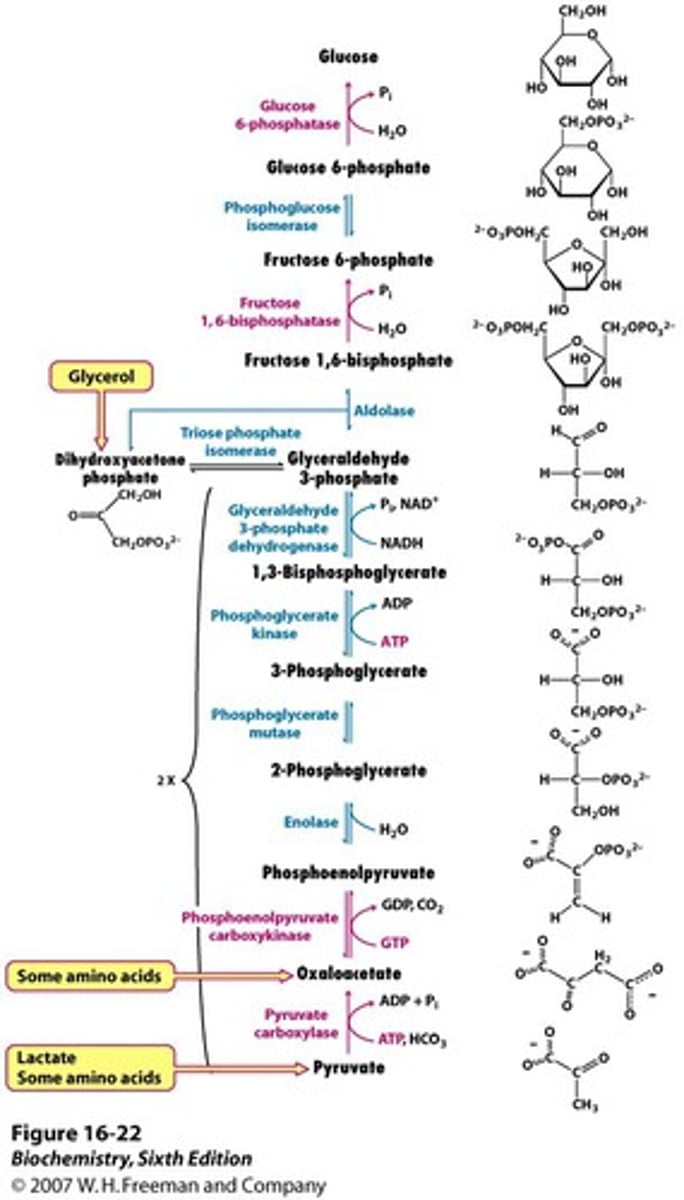
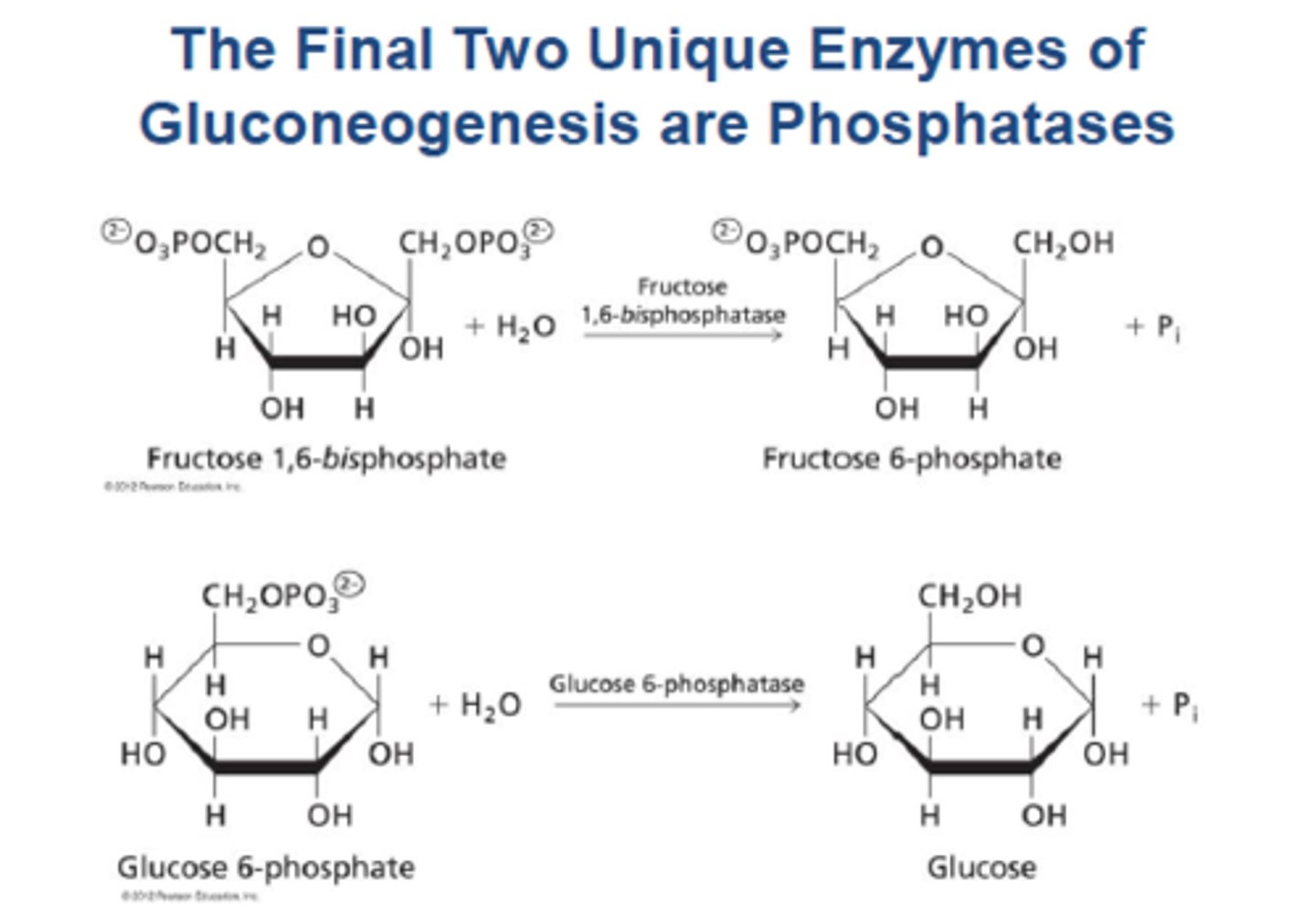
Gluconeogenesis Steps 9 & 10: glucose-6-phosphatase
Glucose-6-phosphatase converts 6phosphate to free glucose, bypassing glucokinase. It is found only in the ER of the liver
acetyl-CoA from fatty acids cannot be converted into glucose, but can be converted into
ketone bodies as an alternative fuel for cells including the brain, thus periods of low blood sugar are usually accompanied by high levels of ketones in the blood
ketone bodies can be thought of as
transportable form of acetyl-CoA that is primarily utilized in periods of extended starvation
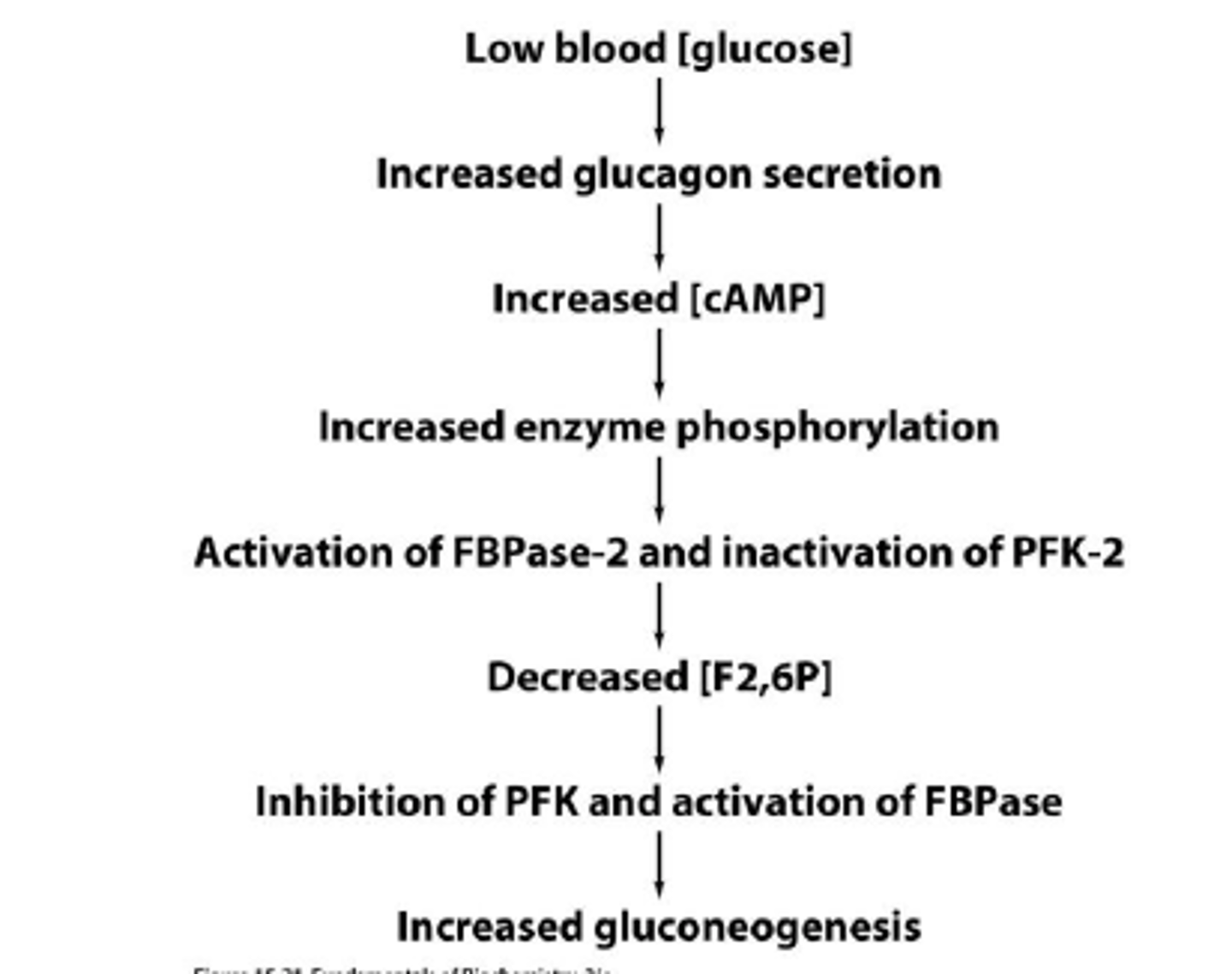
Gluconeogenesis Regulation
Gluconeogenesis linked to fatty acid oxidation?
Because gluconeogenesis requires acetyl-CoA to occur (to inhibit pyruvate dehydrogenase and stimulate pyruvate carboxylase), gluconeogenesis is linked to fatty acid oxidation. The source of acetyl-CoA cannot be glycolysis because this would just burn the glucose that is being generated by gluconeogenesis
Glycolysis/ Gluconeogenesis switch
glycogen
a branched polymer of glucose that is mainly produced and degraded in liver and muscle cells, and functions as secondary long-term energy storage in animal cells.
glycogen stored in cytoplasm as granules, each fragile has central protein core with poly glucose chains radiating outward to form a sphere
glycogen granules composed entirely of linear chains have highest density of glucose near the core
chains are branched, then glucose density highest at periphery of granule allowing more rapid release of glucose on demand
Glycogen stored in liver is a source of glucose moblized netween meals
Muscle glycogen is stored as enrgey reservoir for muscle contractionn
starch
long alpha linked chains of glucose in plants
Glycogen Synthesis
production of glycogen using glycogen synthase (alpha 1-4) and branching enzyme ( a-1,6- linkages)
Liver glycogen is broken down to maintain a constant level of glucose in the blood
muscle glycogen is broken down to provide glucose to the muscle during vigorous exercise
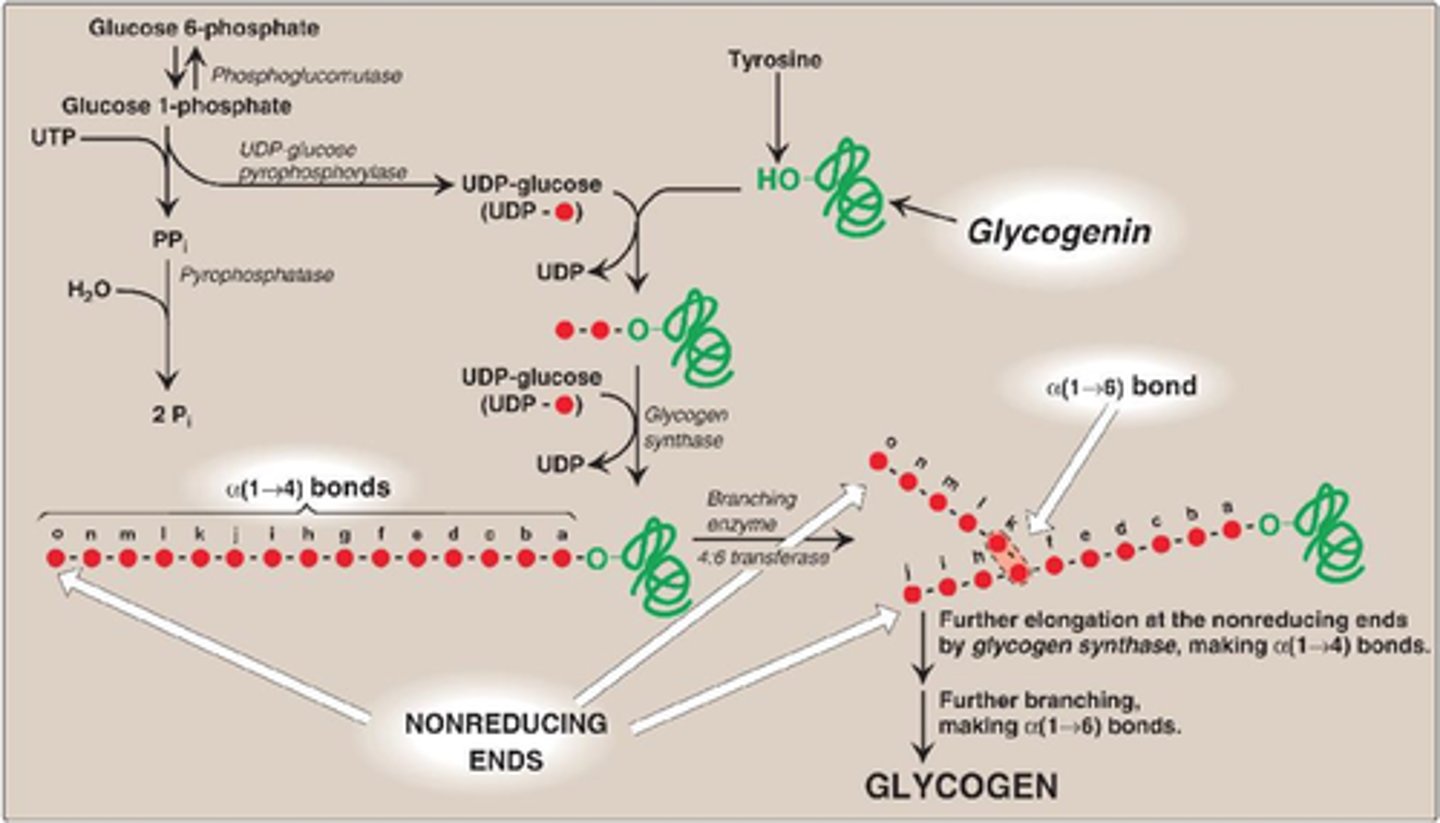
glycogenesis
synthesis of glycogen granules
begins with core protein glycogenin
glucose 6-phosphate converted to glucose 1-phosphate which is then activated by coupling to a molecule of uridine diphosphate (UDP) which permits its integration into the glycogen chain by glycogen synthase. This activation occurs when glucose 1-phosphate interacts with uridine triphosphate (UTP) forming UDP-gluocose and pyrophosphate (PPi)
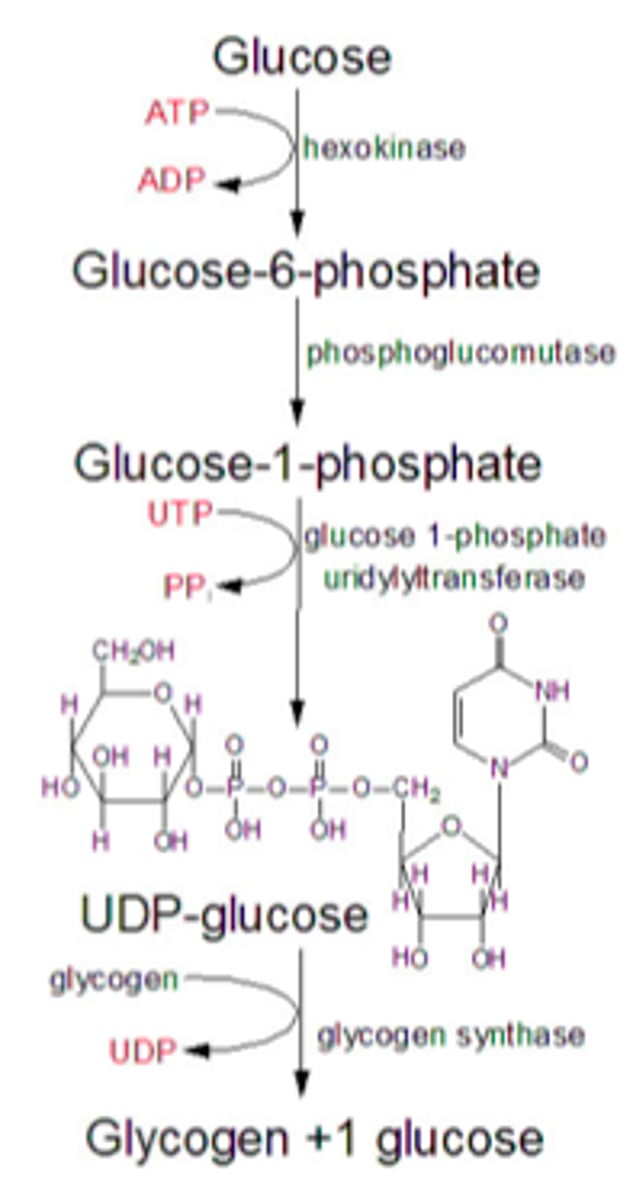
Glycogenesis Key Enzymes
1) Glycogen synthase - crates a-1,4- glysodic linkages between glucose molecules. It is activated by liver and muscle (glucose 6-phosphate and insulin). it is inhibited by epinephrine and glucagon through protein kinase cascade. rate-limiting step of glycogen synthesis. extends both branches
2) Branching enzyme - moves a block of oligoglucose from one chain and adds it to the growing glycogen as a new branch using a-1-6, glysodic linkages. hydrolysis one of thee a-1,4 bonds and forms a-1,6 bond
a-1,4 keeps the branch moving 4ward
a-1,6 (six) puts a branch in the mix
mnemonic 1,4 vs 1,6
alpha-1,4 keeps the same branch moving 4ward, alpha-1,6 puts a branch in the mix
Glycogenolysis
breakdown of glycogen using glycogen phosphorylase and debranching enzyme
phosphorylase breaks bonds using inorganic phosphate instead of water
glucose 1-phosphate formed by glycogen phosphorylase converted to glucose 6-phosphate by the same mnutase used in glycogen synthesis
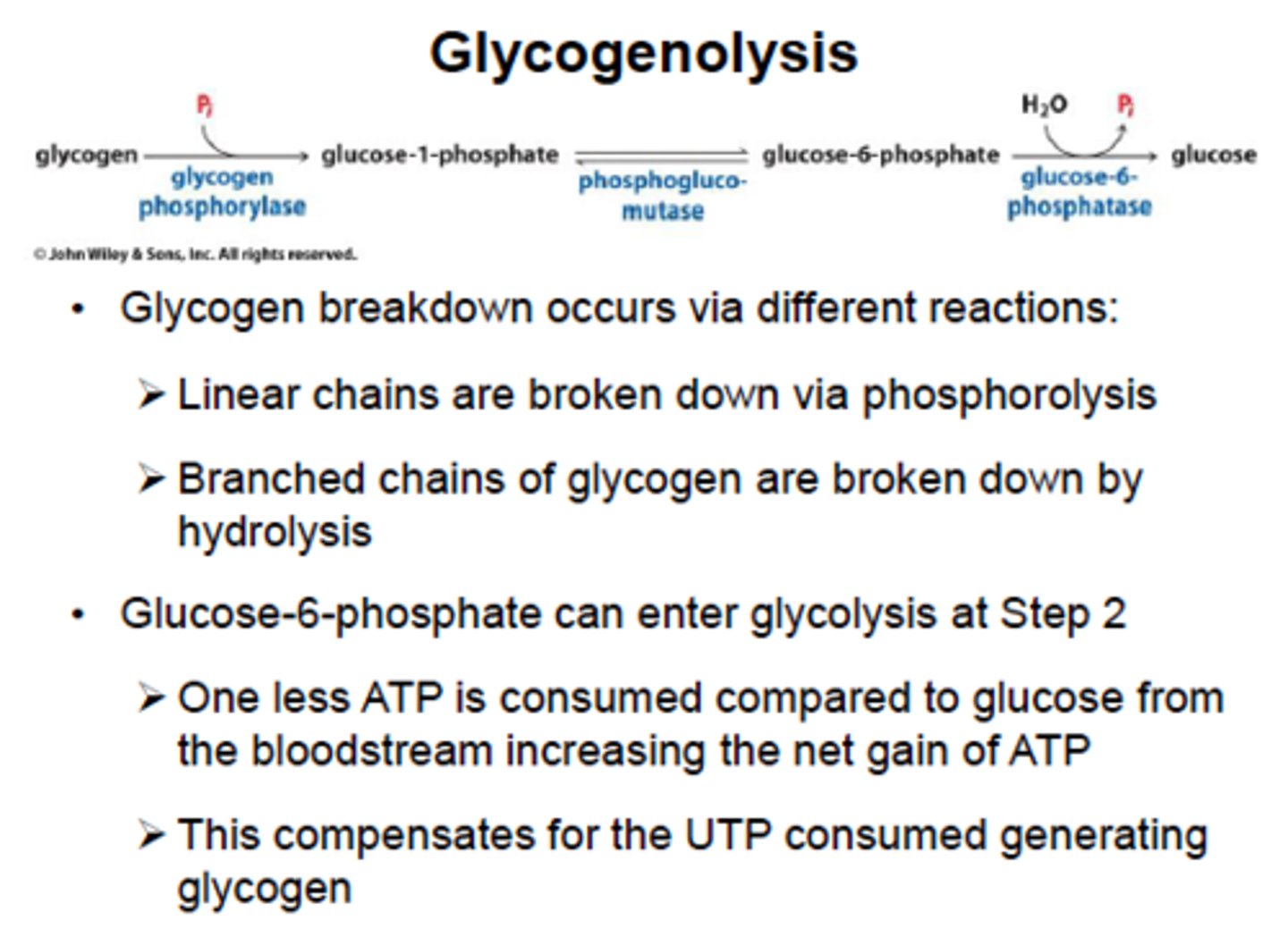
Glycogenolysis Key Enzymes:
1) Glycogen phosphorylase - rate-limiting enzyme, removes a single glucose 1- phosphate molecule by breaking a-1-4-glysodic linkage. In the liver, it is activated by glucagon to prevent low blood sugar, in exercising muscle, it is activated my epinephrin and AMP to provide glucose for the muscle itself. inhibited by ATP
2) Debranching enzyme - moves a block of oligoglucose from one branch and connects it to a chain using a-1-4-linkages. It also removes the branch point, which is connect by a-1-6-links, releasing a free single glucose molecule.

glycogen storage disease
characterized by accumulation or lack of glycogen in one or more tissues
Starch Metabolism
Salivary and pancreatic amylases initiate the hydrolysis of starch during digestion in animals that consume plants. These enzymes cleave the α(1→4) glycosidic bonds of amylose and amylopectin to produce maltose (a disaccharide) and glucose
.
The debranching enzyme hydrolyzes the α(1→6) bonds in amylopectin to ensure complete glucose liberation. This process releases glucose, which is absorbed by cells and can be used for energy through glycolysis, the citric acid cycle, and oxidative phosphorylation.
Other monosaccharides for metabolism?
1) Galactose
2) Fructose
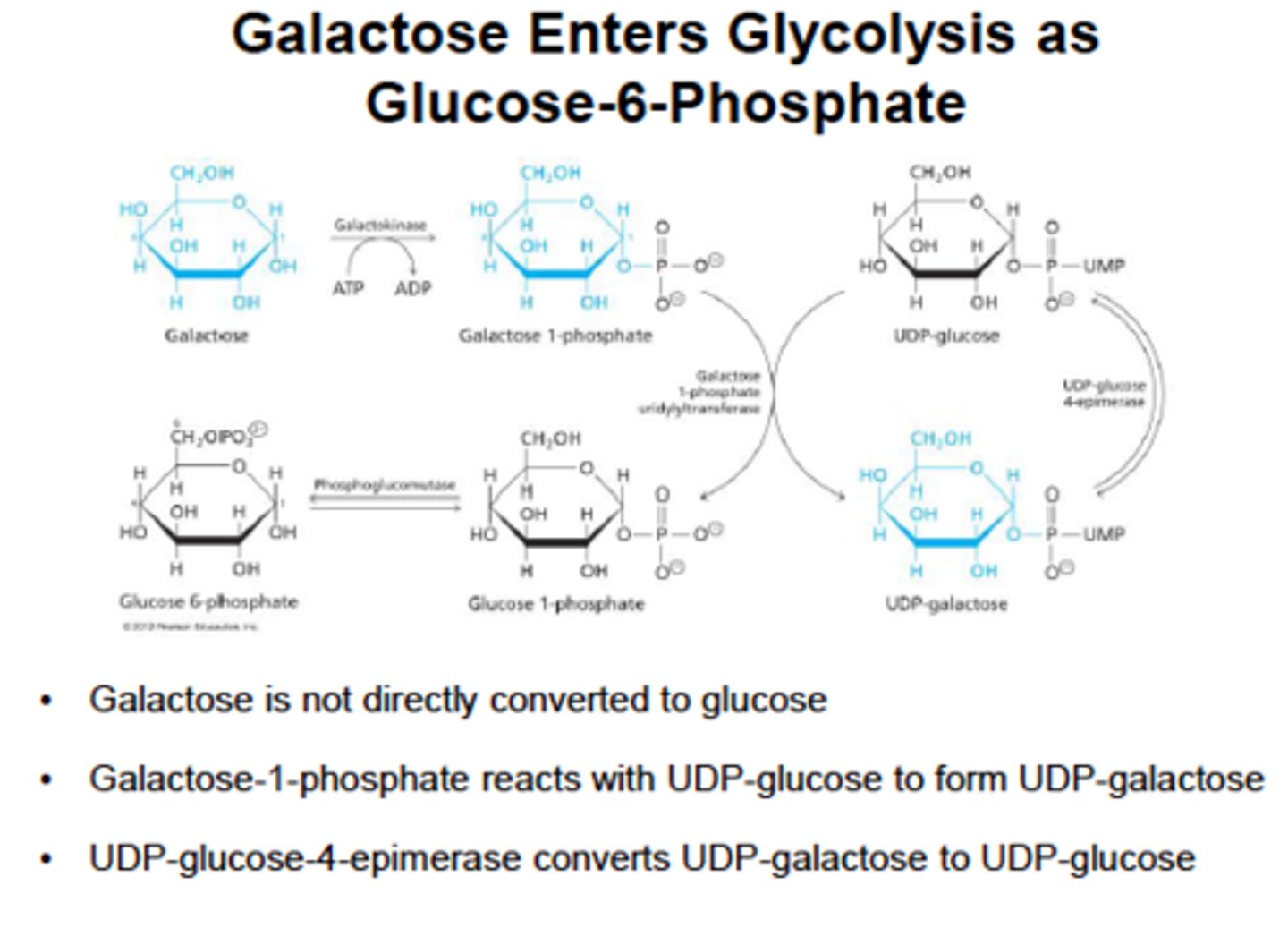
Galactose metabolism
Comes from lactose (lactose -> galactose and glucose) in milk via lactase
1) Trapped in cell by glacatokinase.
2) Converted to glucose 1-phosphate via lactose-1-phosphate uridylransferase and an epimerase.
epimerase- enzyme that catalyze conversions of one sugar epimer to another
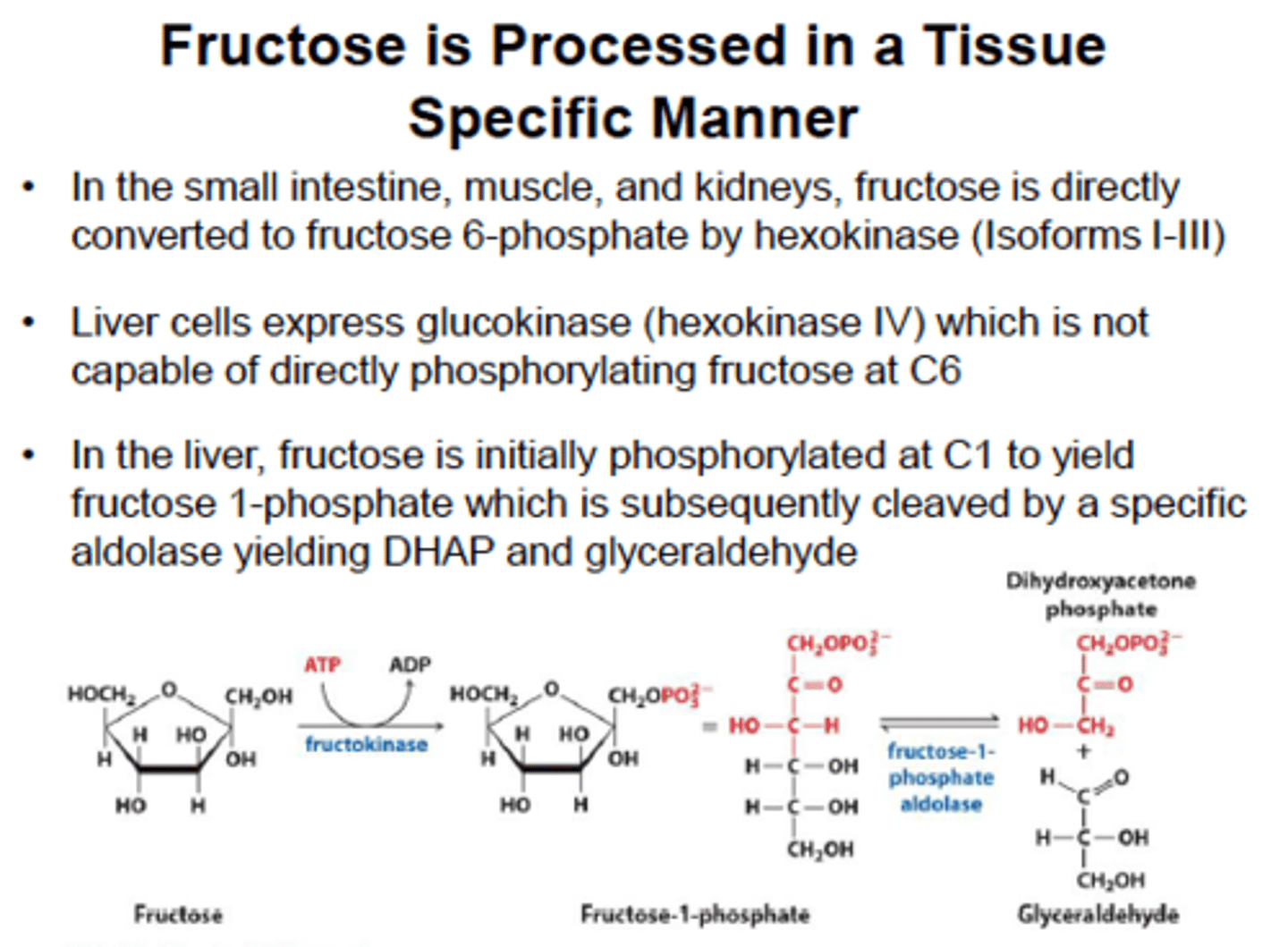
Fructose metabolism
It is trapped in the cell by fructokinase and then cleaved by aldolase B to form glyceraldehyde and DHAP. These products are downstream from the rate-limiting enzyme in glycolysis (PFK-1) , a high-fructose drink supplies a quick source of energy in both aerobic and anaerobic cells
Pentose Phosphate Pathway
AKA the hexose monophosphate shunt.
Occurs in the cytoplasm of most cells, generating NADPH and sugars (ribose 5-phosphate) for DNA biosynthesis.
Uses glucose-6-phosphate to make ribose 5-phosphate
Oxidative phase and non oxidative phase
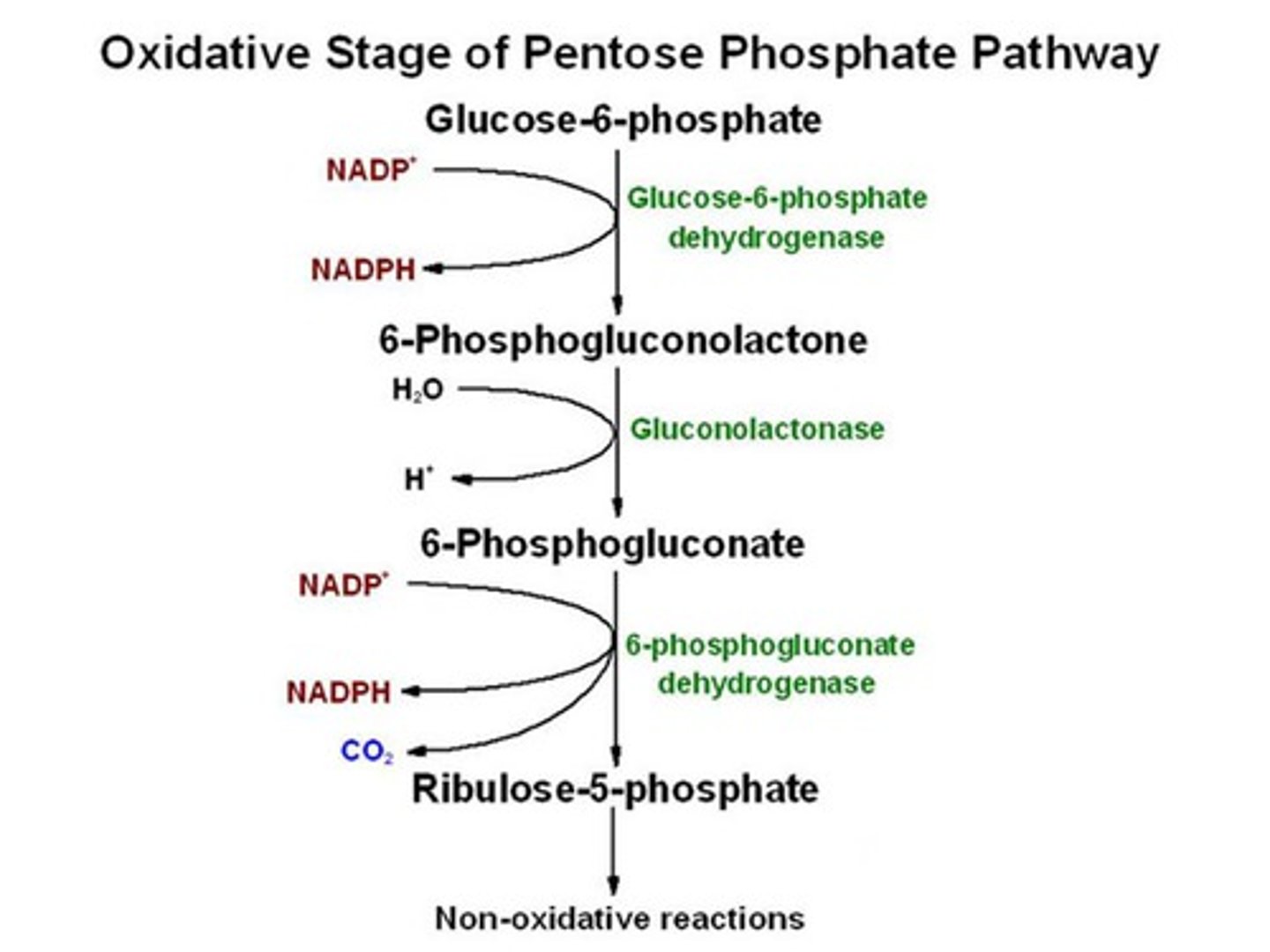
Oxidative phase of PPP
Irreversible;
glucose-6P to ribulose 5-P
Important rate limiting enzyme : glucose-6P dehydrogenase (G6PD)
induced by sugar and NADP+
inhbited by NADPH
Dehydrogenases create NADPH biproduct!

Rate limiting step in PPP?
Glucose-6-phosphate dehydrogenase, which is activated by NADP+ and insulin and inhibited by NADPH.
beings with glucose 6-phosphate and ends with ribulose 5-phosphate and is irreversible
produces NADPH
Non oxidative phase
start with ribulose 5 phosphate and it is a series of reversible reactions to produce equilibrated pool of sugars for biosynthesis including ribose 5 phosphate for nucleotide syntehsis
also fructose 6 phospahte and glyceraldehyde 3-phosphate to feed back into glycolysis; conversely pentoses can be made from glycolytic intermediates without going through the G6PD reaction, these interconversions done by transketolase and transaldolase
Difference between NADH and NADPH
NAD+ is an energy carrier- NADH produced can then feed into the ETC to indirectly produce ATP
NADPH is used in biosynthesis and in the immune system and to help prevent oxidative stress, acts as electron donor, potent reducing agent because ti helps tore molecules be reduced
Functions of NADPH
Acts as the bodies primary reducing agent. Important for:
1) Biosynthesis of fatty acids
2) Assisting in bactericidal activity
3) Maintain supply of glutathione for protection against free radicals.
glutathione
reducing agent that can help reverse radical formation before damage is done to the cell
cellular respiration
Regulation of gllycolsisi
Regukation of glycogensis/lysis
Regulation of gluconegonesis
Regulation of PPP
