Exam 3.3,4,5 Histamines 1, 2, 3
1/95
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
96 Terms
H1 antihistamines are used as _______ agents
H2 antihistamines are ued as _____ agents (which
H1 = antiallergic
H2= antisecretory (gastric acid)
what are some first generation antihistamines?
ethylenediamines
ethanolamine ethers
alyklamines
tricyclic H1 antihistamines
histamine was known to cause __________ in guinea pigs before it was identified physiologically
Where is histamine synthesized?
Where is histamine stored?
anaphylactic shock
synthesized in basophils, mast cells, and parietal cells of gastric mucosa, nueron
vesicles in mast cells
where are mast cells (store histamine) most prevalent?
respiratory tract
skin
blood vessels
are histamines local or systemic?
is histamine metabolized slowly having lasting effects or is it metabolized rapidly?
what effect does histamine have on the cardiovascular system in terms of arteries, and heart rate?
local (histamines are autocoids)
metabolized rapidly
vasodilation of arteries and reflex tachycardia
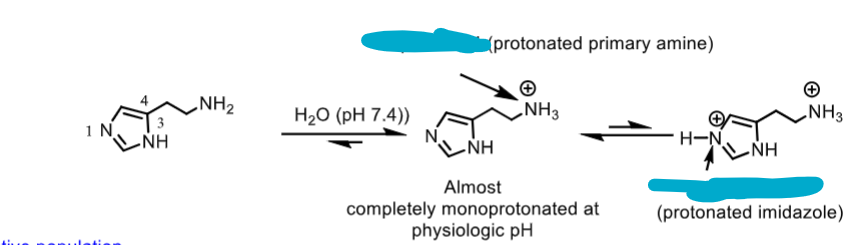
which structures are active?
which is the most membrane permeable?
which are the pH’s of each?
rank from least to most prevelant in physiological pH of 7.4
middle and left protonated structures are active
left unprotonated histamine is inactive
pH of middle protonated amine = 9.4
pH of right prototonated amine AND imidazole= 5.8
histamine least prevalent —> right (both protonated) —> middle (only amine protonated- most prevelant)
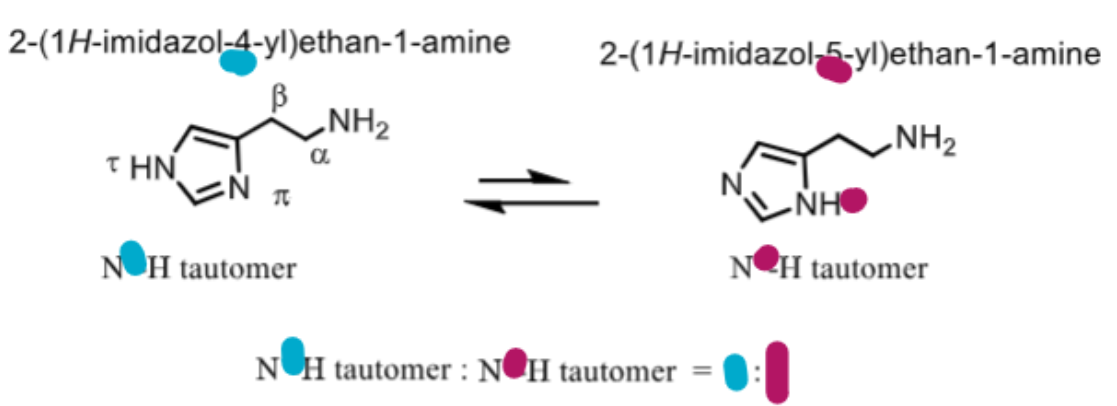
which conformation of histamine is most active and prevalent?
tor (N on imidazole trans to amine) is more active
pi (N on imidazole next to amine) is less active
tor: pi = 4:1
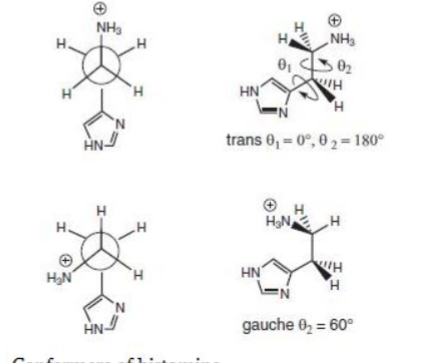
which conformation of histamine is more active?
trans
histamine is synthesized in the _____ _____ of mast cells and basophils by enzymatic _________ of ___________
what is the name of the enzyme and its cofactor?
golgi apparatus of mast cell and basophil through decarboxylation of L-histidine
enzyme= L-histidine decarboxylase
cofactor = vitamin B6 (pyridoxal)
histidine is rapidly metabolized once released, what are the two different way its metabolized?
which enzymes are responsible for each?
N-methylation of N in imidazole ring (N-methyltransferase)
then
Oxidation of primary amine to aldehyde (monoamine oxidase B and diamine oxidase) then aldehyde —> N-methyl imidazole acetic acid (aldehyde oxidizing enzymes- ALDH, XO, ADO)
histamine—> aldehyde (MAO/DAO) —> imidazole acetic acid (ALDH, XO, ADO - aldehyde oxygenase) —> IMIDAZOLE ACETIC ACID—> IMIDAZOLE ACETIC ACID RIBOSIDE (PRT) — ribosylation
histamine is stored in ______ _______ in mast cells as a complex with ________ and in the basophils in the blood as a complex with ________
histamine release occurs when _______ bind to __________
secretary granules in mast cells as complex with peptidoglycan
basophils in blood as complex with chrondroitin
histamine is released when allergens bind to igE with mast cells bound to them
The sequence of events in immediate hypersensitivity that leads to histamine release:
initial contact with an antigen leads to specific _____synthesis by ___cells
secreted _____ binds to mast cells or basophils through high affinity ______ receptors
when mast cells with ____ now attached come into contact with an antigen an immediate hyersenitivity reaciton is triggered by cross linking the ___ molecules resulting in release of histamine and other inflammatory mediators
IgE antibody synthesized by B cells
IgE binds to Fce receptors on mast cells
when mast cell with IgE comes into contact with antigen histamine is released from the mast cell
which histamine receptors are coupled to
Gs (cAMP) vs Gq (PLC) ?
Gs = H2, 3, 4
Gq = H1
what is the difference between inverse agonists and neutral agonists?
inverse agonists decrease the intrinsic activity that the histamine receptor has on its own BUT DONT impact histamine binding
neutral agonists prevent binidng of histamine receptors BUT DONT impact intrinsic activity
a prerequisitite for an ________ agonit repose is that the receptor must have a constituitive/intrisic/basal level activity in the absene of any ligand
inverse agonist
_______: increases the activity of a receptor above its basal/intrinsic level
_______: decreases the activity below the basal/intrinsic level
_______: has no activity in the absense of an agonist or inverse agonist but can block the activity of either
ALL H1 and H2 ANTIHISTAMINE THAT WE DISCUSS IN THESE LECTURES ARE ______ ________
agonist
inverse agonist
neutral antagonist
all H1 and H2 receptor we discuss are inverse agonists
______ was derived from tooth pick weed AMmi Visnaga and had bronchodilatory effects
Even though ______ was developed based on this plant derived structure it could not reverse antigen-induced bronchiolar constriction BUT it could prevent bronchospasm. Why?
khrelin
chromolyn
mast cell stabilizer such as cromylyn simply prevent MORE histamine from being released through the activation of PLC and phosphorlylation of the moesin (78kd) protein. They cant reverse the damage that was already done, but they can prevent further constriction leading to bronchospasms by by preventing further histamine release
Do mast cell sstabilizers derived from khellin such as
cromolyn sodium
nedocromil sodium
lodoxamide
pemirolast potassium
block histamine receptors?
NO they dont prevent histamine from binding to receptors they prevent histamine from being released AT ALL by phosphorylating moesin proteins that allow for exocytosis
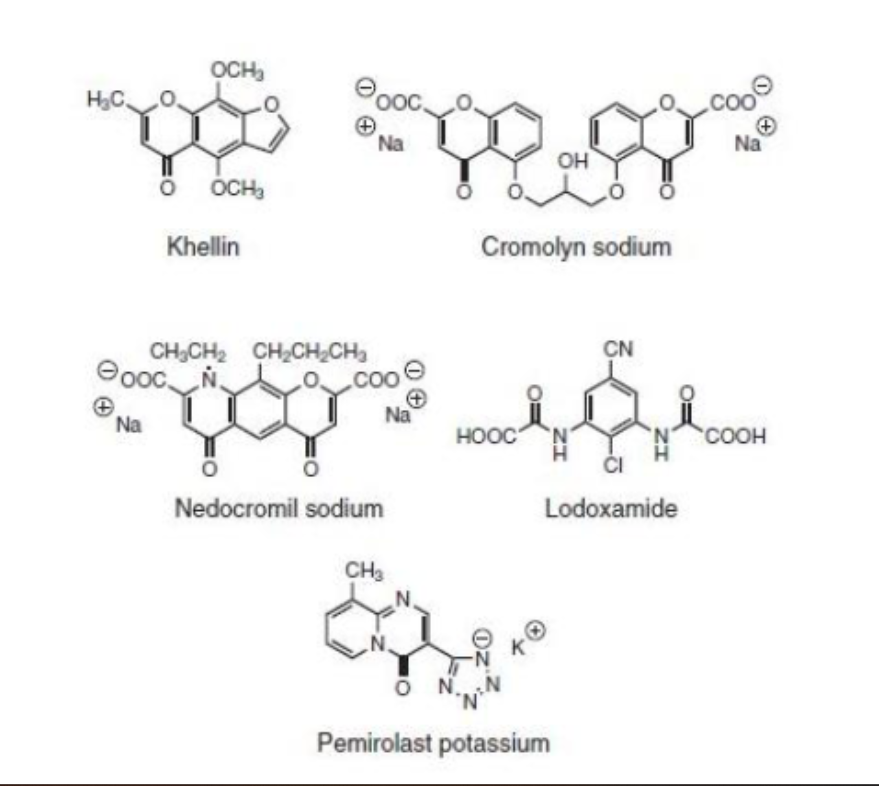
all mast cell stabilizers have either one of two functional groups, what are they?
acidic COOH
acidic tetrazole (pemirolast- COOH surrogate)
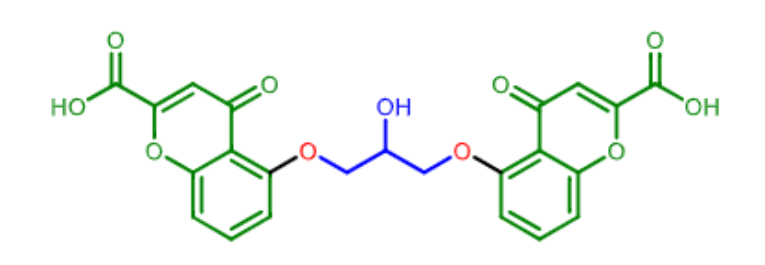
which molecule is this?
it is a _______ derivative
what is its mechanism of action?
cromolyn
chromenone derivative
mast cell stabilizer (prevent histamine release)
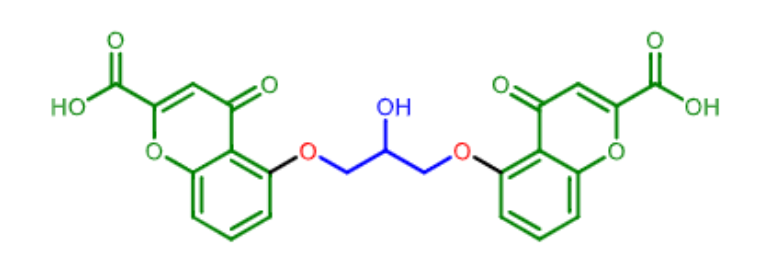
Cromolyn:
what is this mast cell stabilizer used for?
what dosage forms can it be used in?
does it have good oral bioavailability?
bronchial asthma (inahled powder or nebulizer)
prevention of exersize induced bronchospasm and allergic rhinitis (nasal spray)
conjunctivitis and keratitis (eye drops)
mastocytosis- skin disease due to high mast cell levels (orally)
BAD oral bioavailability (less than 1% !)
inhalation allows for 8% to reach lungs
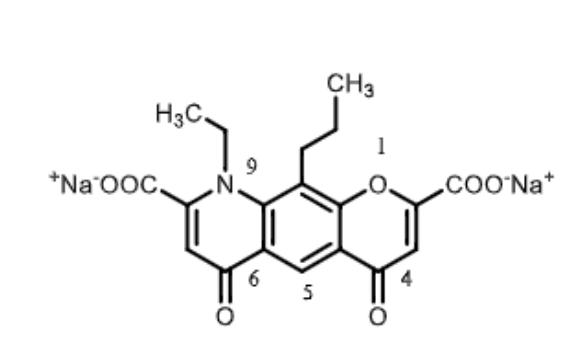
Nedocromil
It is a ______ derivative
what is its moa?
what are its medical uses?
chromenone derivative
mast cell tabilizer
allergic CONJUCTIVITIS
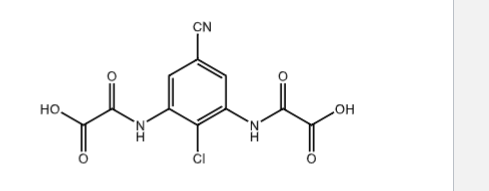
ladoxamide
moa?
medical use?
________ marketed as ___ of tris9hydroxymethyl)amine (COCH2)3C-NH2
moa: mast cell stabilizer
for: ocular disease
dicarboxylic acid , salt
First Generation Histamines:
what was the first antihistamine to be used and what was it used for?
what are 1st gen antihitamines used for? what are some off target activities?
what are some side effects?
short acting first generation antihistamines can be used as _______
piperpoxan used to treat bronchospasm in guinea pigs
allergic responses
off target:
cholinergic
adrenegic
dopaminergic
seretonergic
side effects:
CNS: sedation, drowsines, somnolesene
anticholinergic: blurred vision, dry mouth, urinary retention, constipation
apetite supression, muscle spasm, anxiety, irritability, tremor, TACHYCARDIA
short acting 1t gen antihistamine- OTC sleep aid
what effects do first gen antihistamines have on the following systems
cholinergic
a-adrenergic
seretonin
histamine H1 receptor
cholinergic (anticholinergic effects)
constipation / urinary retention
dry mouth
sinus tachycardia
a- adrenergic
hypotension
dizziness
reflex TACHYCARDIA
seretonin
increase appetite
H1 receptor:
decreased
inflammation
neurotransmission in the CNS
cognitive psychomotor performance
increased
sedation
appetitie
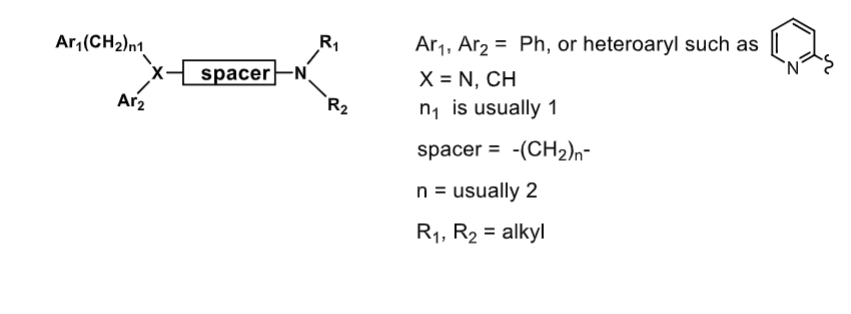
the following is the general structure of first gen antihistamines
Ar1 and Ar2 usually ________
X connecting both is usully ____ or ____
(CH2)n1 before Ar1 usually ___
spacer is usually - CH2 (____)
R1 and R2 are ____________
Ar1 and Ar2 usuall Ph or heteroaryl
X usually N or CH
1
spacer typically 2 CH2
R1 and R2 = alkyl
Ethylenediamine H1 Antagonists:
____ class of antihistamines
are they still used?
which side effects are common?
how can they be metabolized?
first
some
CNS sedation side effects
metabolized by
N-demethylation
deamination
N-glucoronidation
what are examples of ethylenediamine H1 antihistamines?
phenbenzamine
tripelennamine
methapyrilene
thonzylamine
antazoline
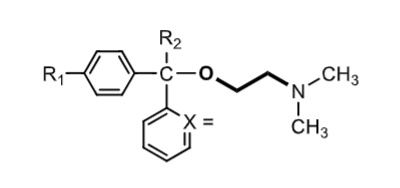
ethanolamine ethers
X=
R2 =
R1=
when the benzylic carbon is chiral two enantiomers result
which enantiomer is more potent?
x= CH or N
R2 = H or CH3
R1= H, Cl, Br
S enantiomer > R enantiomer
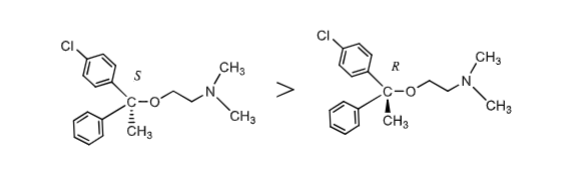
which enantiomer of ethanolamine ethers is more potent?
S enantiomer
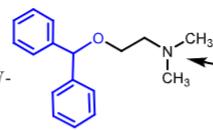
Diphenhydramine:
__________ first gen antihistamine
oldest and still widely used
found in ________
how is it adminsitered?
side effects:
if has significant _________ activity which is repsonsible for _________, _________, ___________, _________
these side effects can actually be used to treat _________
also has anti- ________ properties
causes CNS ________
ethanolamine ether
benadryl
orally
side effects:
anticholinegic (dry mouth, tachycardia, blurred vision, urinary retention)
anticholinergic side effects can treat parkinsons disease (deccreased dopamine increases aceytlcholine - diphenhydraine can decrease effect)
has anti-emetic properites
CNS depression
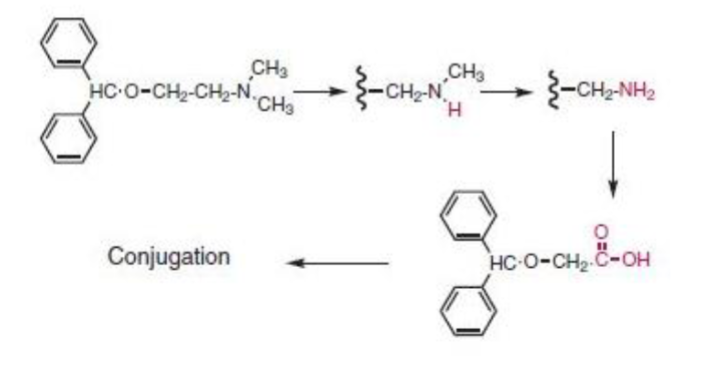
metabolism of diphenhydramine:
sequential ____ _________ to form ____ amine (minor amounts due to short half life)
subsequent ________ to form a ______ acid metabolite (major metabolite!)
________ conjugates are also seen
N-dealkylations to form primary amine (minor)—>
deamination to form carboxylic acid (major)—>
carboxylic acid conjugates also seen
Dimenhydrinate (Dramamine):
a salt of diphenhydramine with _______(1:1 molar ratio) used for treatment of _____________
inhibits _________ trigger zone (CTZ) which stimulates the _____ center within the CNS
diphenhydrinate has a _______ effect on hyperstimulated
______ is a stimulant that REDUCES SEDATION CAUSED BY diphenhydramine (oral dose: 50-100mg every 12 hours)
diphenhydramine + 8-chlorotheophylline treat motion sickness
inhibits chemoreceptor trigger zone —> vomiting zone —> antiemetic
depressent effect
8-chlorotheophyllline
Clemastine Fumarate:
is NOT a _______ dertiivative since it has 3 carbons between the nitrogen and oxygen atoms
how many possible isomers are there? which is the most active?
is it more or less potent than diphenhydamine? (1-4 mg every 12 hours)
why does it have less sedation?
NOT ethanolamine
4 isomers —RR most active!
more potent than diphenhydramine
less sedation bc/ more potent so less given per dose
Clemastine Fumarate:
what gen antihistamine is it?
what are its uses?
what are side effects?
how is it metabolized?
1st gen antihistamine
used for allergic rhinitis, mild urticaria + angioedema in pediatric patients
intraocular pressure + CNS sedation
O-dealkylation —> alcohol dehydration
Alkylamine H1 antihistamine:
most widely used OTC antihistamine until 2nd gen antihistamines
has greater selectivity vs ______ and ______ receptors
which enantiomer has a higher affinity for the H1 receptor by 200-1000 fold?
are they more or less CNS penetrant than ethyldiamines and ethanolamine ethers?
do they have longer/shorter duration of effect?
how are they metabolized?
selective vs muscarinic and adrenergic receptors
S enantiomer more selective for H1
less sedative than ethyldiamines and ethanolamine ethers
longer halflife and duration of action
N- dealkylation by CYPs (2ndary and primary amines in plasma)
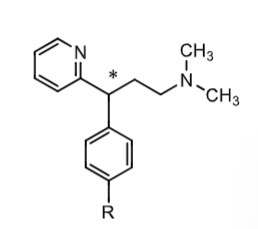
what are some examples of alkyamine H1 antihistamines?
what would R be for each?
Pheniramine = H
Chlorophenirarmine = Cl
Dexchlorpheniramine = Cl
Dexbrompheniramine = Br
Propheniramine = Br
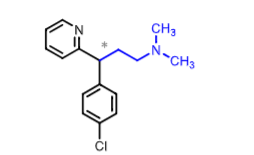
Chlorpheniramine:
more potent than ______ and ____
which enantiomer is more active
what is it used for?
what are some adverse effects?
how is it metabolized?
ethanolamine ethers and ethylendiamines derivatives (except clemastine)
Racemic BUT S more active (dexchlorpheniramine)
used for cold, flu, allergy symptom relief
AE: diziness, fatigue, despression, blurred vision + HYPOTENSION
metabolized: N-dealkylation (significant first-pass)
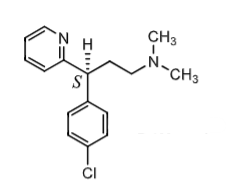
Dexclorpheniramine:
more active and selective S- enantiomer of ____________ (racemic)
S enantiomer has greater affinity for H1 receptor compared to ______ and ________
what are adverse effects?
how is it metabolized?
Chlorphenaramine
S more selective for H1 compared to muscarinic and adrenergic (so less sedation)
adverse effect: diziness, fatigue, depression, nervousness
metabolism: N-dealkylation
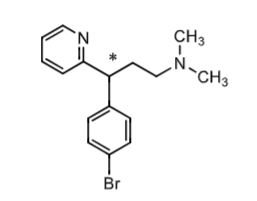
Bropheniramine:
simmilar to _______________
available OTC
what is it used for?
how is it metabolized?
what are some adverse effects?
chlorphenamine
used for syptomatic relief of common cold, flu, allergies
metabolism = N-dealkylation
adverse effect:
COPD/asthma: may reduce the volume and cause THICKENING of bronchial SECRETION resulting in obstruction of respiratory tract
CNS: diziness, fatigue, depression, nevousness
What is the extent of ionization (>50% or <50%) of histamine ring nitrogen (pKa=5.8); side chain amine (pKa = 9.4)?
at pH = 7.4
nitrogen is less than 50% ionized (pka=5.8)
amine is more than 50% ionized (pka= 9.4)
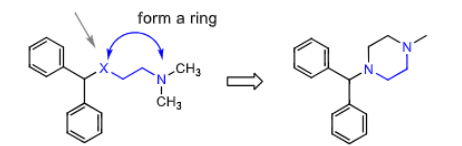
Piperazine H1 antihistamine:
considered a constrained analog of ______________ (same number of atoms between the two hetero atoms
what are they used to treat? how does it work?
ethanolamines
treat motion sickness and vertigo by inhibiting the chemoereceptor trigger zone (CTZ) in CNS which stimulates vommiting center
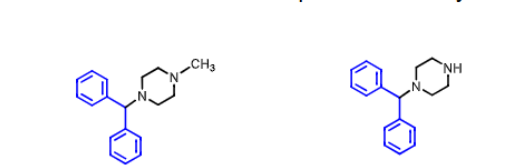
Cyclizine (hydrochloride, Marezine)
what is it used for
has significant _____ properties (adverse effect)
teratogenic in rodents, how about humans?
is its metabolite on the right active? how is it metabolized?
used as anti-emetic
has anticholinergic properties (dry mouth, tachycardia, urinary retention)
No cant cause cancer in humans
NO NORCYCLIZINE - demethylated metabolite is inactive (by CYP2D)
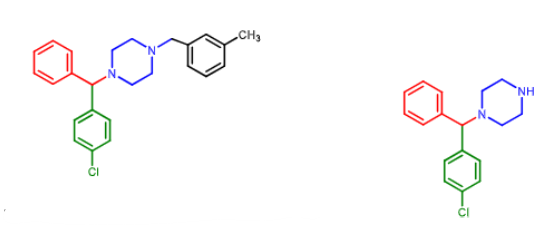
Meclizine:
available OTC
used to prevent ________ and _________
how is it metabolized?
vertigo and motion sickness
debenzylated to nor-chlorcyclizine by CYP
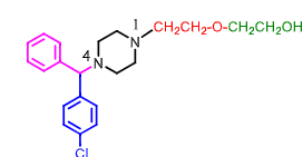
Hydroxyzine:
HIGHLY ________ prescription drug
used more so as a _______ and _______then for allergies
metabolized into ________ by CYP
what are some side effects?
HIGHLY SEDATIVE (tranquilizing!)
used for anxiety and anti-emetic
metabolized to cetirizine
side effect: QTC prolongation, drowsiness, respiratory depression
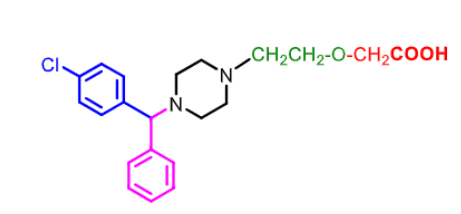
Ceterizine:
racemic!
does it have sedating effects?
______ _____ metabolite of ________
due to the presense of the _____ _____ group it is very POLAR AND DOES NOT CROSS BBB
LEAST sedating H1 antihistamine
CARBOXYLIC ACID metabolite of hydrozyzine
bc/ of carboxylic acid it is polar does not cross BBB and is less sedating
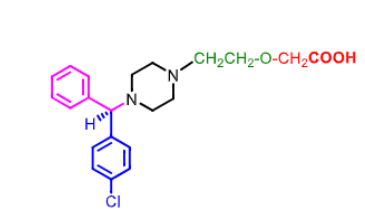
Levocetirizine
___ enantiomer of _________
which enantiomer is more potent?
do the R and S enantiomers interconvert readily in vivo
are there any PK diffrences?
what is it used for?
R enantiomer of cetirizine (racemic) which is caboxylic acid metabolite of hydrolyzine
R enantiomer is more selective for H1 then dextrocetirizine
enantiomers DONT interconvert readily
NO PK differences between lecvocetirazine and cetirazine
chronic rhinitis and idopathic uticaria (hives)
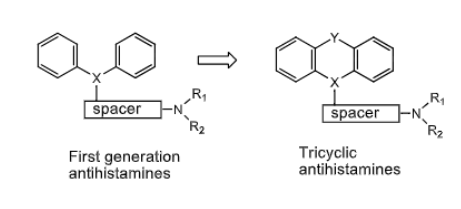
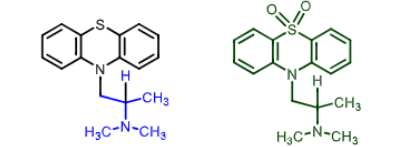
Tricyclic H1 Antihistamines:
what can X and Y be ?
X= C, CH, N
Y= CH2, S, O, NH, CH2O, CH2CH2, CH=CH
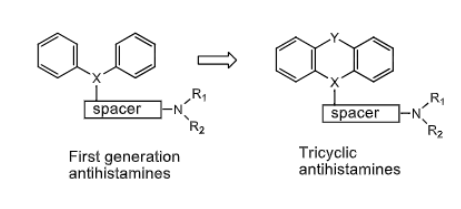
Tricyclic H1 Antihistamines:
most have ________ effects
long/short duration of action
used as ________ agents to treat _________
also can treat ______ and __________
sedative
long
used as pruritic agents to treat urticaria (hives)
can treat nausea and vomitting
Promethazine (Phenergan)
Black Box Warning: DONT use in pediatric patients younget than ____ years because of potential risk for ____________
can it be found OTC?
does it have sedative effects and is it slow or fast acting?
can be used as an anti________ for nausea and vomitting and can potentiate effects of ______ agents due to ____ effect
how it it metabolized to the sulfone on the right? is the metabolite acitve?
dont use younger than 2 bc/ of respiratory depression
NOT OTC
sedative effect yes and it is long acting
can be used as antiMUSCARINIC for nausea and vomitting
potentiate analgesic effect due to sedatitve effect
N demethylation and aromatic hydroxylation can lead to sulfone
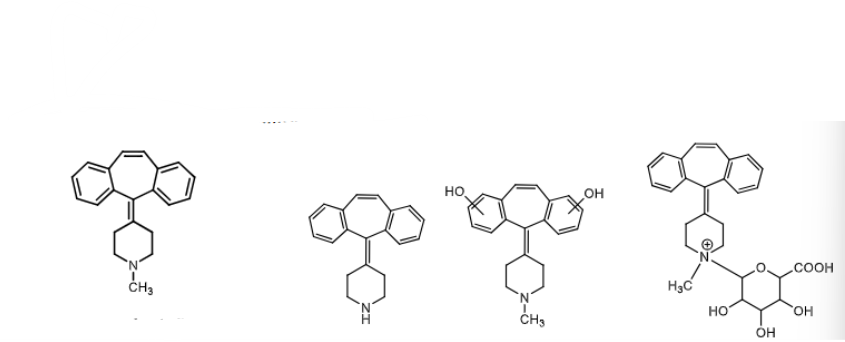
Cyproheptadine:
has significant ___________ and ______________activity
is it sedative and how long/short does it act?
can ALSO be used as an ________ due to its anti ______ activity
how is it metabolized, are any metabolities active?
antiSEROTONIC and anticholinergic activity
sedative and long acting
due to its antiseretonic acitivity it can be used an ANTI-ANOREXIANTS and CACHEXIA (seretonin supresses appetite)
metabolites are inactive (N-demethylation, aromatic hydroxylation, N-glucoronide)
Second Generation H1 Antihistamine:
what allows them to be less sedating than first generation?
how are their half lives?
they are zwitterionic which prevents them from crossing BBB, also substrates for Pgp if they do get to the brain they will be kicked out
long half lives allowing for once a day dosing
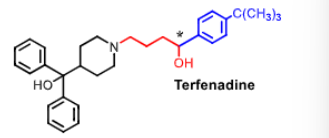
Terfenadine - NO LONGER USED
racemic!
first _______ generation antihistamine
why was it removed from the market?
2nd generation antihistamine
dangerous cardiac arrythmias (QT interval prolongation) when taken w/ drugs metabolized by CYP3A4 (increased levels due to competition) —> torsades de point
QT prolongation = block of hERG (human ether a go go ) alpa subunit of cardiac K+ channel
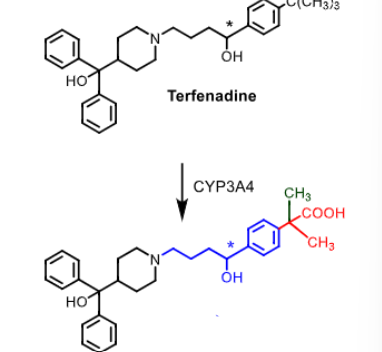
Terfenadine is metabolized to ___________using ___________
terfenadine metabolized to fexofenadine using CYP3A4 through carboxylation
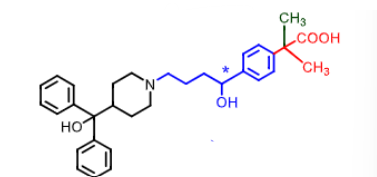
Fexofenadine:
____ generation antihistamine
what makes fexofenadine non-sedative?
does it also inhibit hERG channel like terfendine?
how many times can it be taken a day?
second
nonsedative due to carboxylic aci dbeing too polar to cross BBB and basic amine (zwitterion) AND kicked out by pgp
NO does not inhibit hERG so does not cause cardiac arrythmia
taken once a day orally
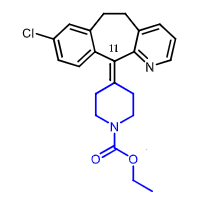
Loratadine:
____ gen antihistamine
can it be found OTC?
what allows it to have less sedating effects?
what is it used for?
does it inhibit hERG channel?
how often is it taken?
is it a prodrug?
how is it metabolized?
2nd gen antihistamine
YES OTC
effluxed out by pgp in brain - allows to be nonsedating
used for allergic rhinitis and urticaria
does NOT inhibit hERG like terfenadine
taken ONCE a day
loratadine (prodrug) metabolized by CYP2D6 and CYP3A4 to desloratadine (active)
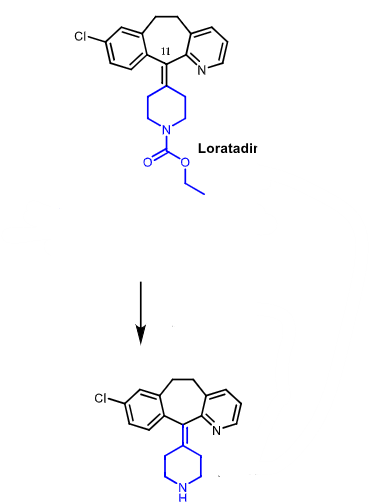
loratadine is a prodrug metabolized to __________ by ________ and ________
loratadine —> desloatadine using CYP3A4 and CYP2D6
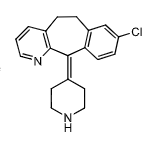
Desloaratadine:
active metabolite of __________ indicated for __________ and _____________
further metabolized through ________ are these metabolites active?
active metabolite of desloratidine used for idiopathic uticaria and allergic rhinitis
further metabolized to hydroxydesloratidine through hydroxylation (active)
Cetirizine:
is it considered a 1st or 2nd gen drug?
is it sedating, why or why not?
PARTIALLY sedating
1st gen but least sedating of them all bc/ it has a carboxylic acid zwiterrion and pumped out by pgp
TOPICAL H1 antihistamines:
used for _________________
the density of mast cells in the ________ is high and the histamine concentrations in tear film are significant in the ocular allergic response
application relieves _________,____________, and _________
topical = conjuctivitis
mast cells in conjuctiva (mucous membrane of eye)
relieves itchiness, congestion of conjuctiva, and erythma
which H1 antihistamines are topical?
olaptadine
emedastine
ketofen
betoplastine
levobastine
azelastine
epinastine
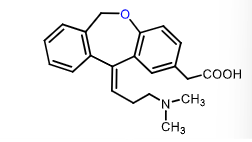
Olopatadine:
What are its dual MOAs?
what is it used to treat?
does it have a rapid/slow onset?
does it have long/short duration of action?
why does it have limited tissue perfusion?
H1 topical antihistamine AND mast cell stabilizer (prevent release of histamine, tryptase, and PGD2)
conjuctivitis (nasal spray and eye drops)
rapid onset
long duration of action (slow disasociation from receptor) — like most tricyclic antihistamines
it is zwitterionic at pH 7.4 —> limited tissue perfusion
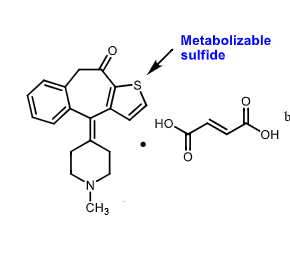
Ketofin:
Dual MOA: what does it do?
approved for ___________
______ metabolite
antihistamine AND stabilizes degrandulation of EOSINOPHILS
allergic conjuctivitis of eyes
sulfur metabolite
what are the 6 causes of peptic ulcer?
what are some treatmnet options
INSUFFICIENT MUCOSAL DEFENCE
h.pylori infection
increased acid secretions
long term use of NSAIDs
stress
genetisc
tx: eradicate h.pylori, CONTROL STOMACH ACID SECRETION, use cytoprotective agents
Mechanism of Stomach Acid Secreiton:
what are the 3 mediators that regulate the secretion of gastric acid from parietal cells? What do all three of them do?
histamine (H2 receptor)
gastrin (gastrin receptor)
aceytlcholine parasympathetic stimulation (M3 receptor activation)
activate H+/K+ ATPase that releases H+ in exachange for K+
H2 antihistamines are used in the treatment of __________ and __________
what role does pepsin play?
antisecretory agents are also included in multidrug treatmnet of eradicating _______
can antacids be used as treatment?
peptic ulcers and gastroesophageal disease (GERD)
low pH due to increased acid—> activate pepsin
antisecretory agents used to eradicate H.Pylori in peptic ulcers
antacids CANNOT be used to treat GERD or peptic ulcers bc/ there is TOO MUCH acid
what are the two ways to pharmacologically decrease stomach acidity?
H2 inhibition
blocking actual proton pump (H/K ATPase) hat histamine, gastrin, acytelcholine activates!
H2 histamines are _________agonists/antagonists
inverse AGONISTS (decrease BASAL activity of receptors not binding)
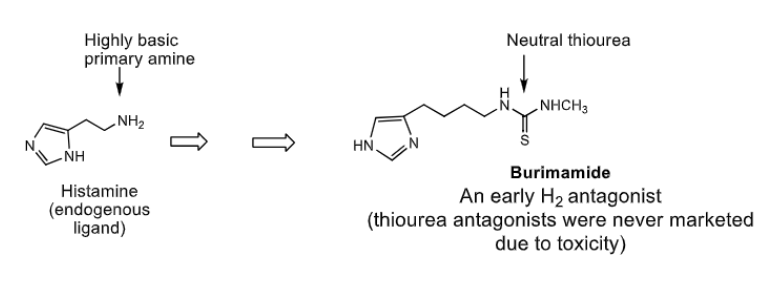
H2 antihistamines are design based on structure of _________
the highly basic primary amine of ________ is replaced by NON-BASIC ___________ (due to toxicity safer bioesters were later used)
histamine
highly basic primary amine —> non basic THIOUREA (toxic!)
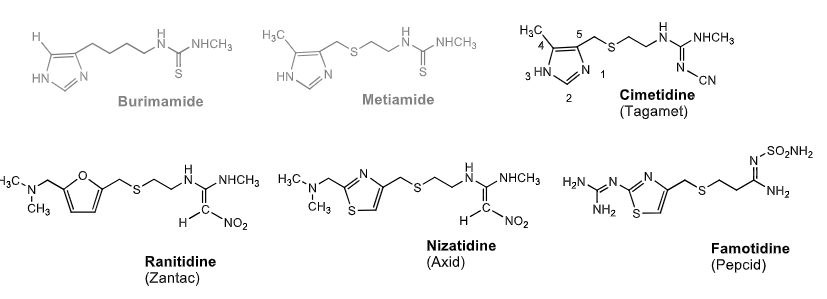
what are the three structural requirements of all H2 antagonists?
5 memebrered heteroaryl
4 atom side chain
POLAR NONBASIC urea isostere
which two H2 histamines were never marketed due to their toxicity and pure toxicity?
burinamide and metiamide
H2 antagonists:
________: contained 4,5 imidazole linked to side chain w/ N-cyananoguanidine. Sulfure on side chain increases _______
________: contains furan ring. nonbasic polar group = 1,1 diaminonitroethane
________: replaces furan eith thiazole everything else same
________: thiazole directly attached to guanidine. side chain attached to aminosulfamoylamine
Cimetidine (4, 5 imidazole N-cyanoguanidine + sulfur) sulfure increases POTENCY
Rinantine (furan + 1,1 diaminonitroethane
Nizatidine (same except furan—> thiazole)
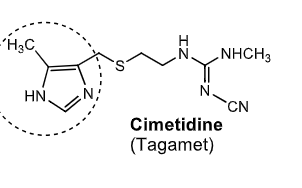
Cimetidine:
first _________ discovered
how is bioavailability?
CYP450 inhibitor/inducer
used to treat
H2 antihistamine
50% bioavailability
inhibitor
treats gastric ulcer, GERD, hypersecretory conditions
what are some side effects of cimetidine?
which structure allows for CYP inhibition?
somnolence + confusion
gynecomastia (bc/ increased prolactin reaction to low ph)
inhibition of renal tubular secretion of drugs including procainamide
DDI with drugs that need CYP metsbolism (phenytoin, theophylline, benzodiazepines, warfarin, quinidine, beta-blockers, calcium channel blockers etc)
imidazole of cimetidine —> inhibition of CYP
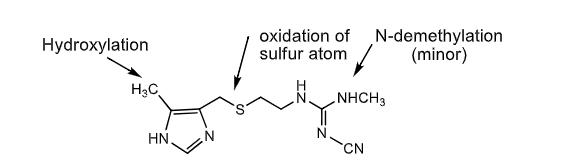
the following are metabolic pathways of which drug?
hydroxylation (CH3 on left end coming out of ring)
oxidation of sulfur
n-demethylation (on right end)
H2 antihistamine antihisamine
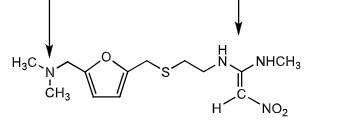
Rantidine:
which group is basic and which is non basic?
how it its biovailabiltiy?
subject to ________ metabolism. which organ does the most metabolism?
it does NOT stimulate pituitary so no symptom of _________
what is it used to treat?
which metabolism pathwats can it take?
basic group on left (protonated @ pH7) non basic group on right like all H2 inhibitors
50% bioavailability
first pass (eliminated primarily through kidney) - kidney
gynecomastia
treats GERD , peptic ulcers, hypersecretions
S-oxide formation and N-demethylation
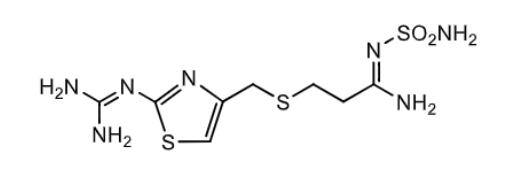
Famotidine (pepcid):
most _______ H2 antihistmaine (even more than cimetidine?)
how is its oral bioavailability?
to what extend does it go through first pass metabolism?
what is its major metabolite, and is it active?
what is it used to treat?
potent
50%
little first pass metabolsim
major metabolite = S-oxide and NOT active
duodenol ulcer, gastric ulcer, GERD
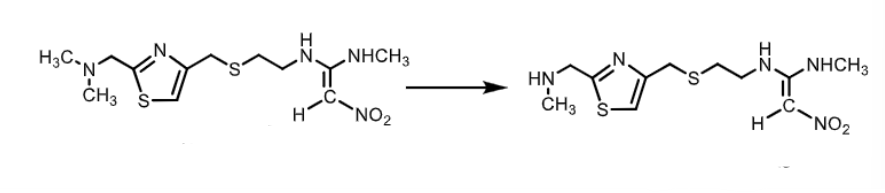
Nizatidine:
how is its oral bioavailability?
does it effect CYP450 enzymes?
does it have an active metabolite?
is nitazidine a prodrug?
what is it used to treat?
90% ! greater than cimetidine and famotidine (50%)
NO effect on CYP450
YES (N-desmethyl)
YES prodrug
duodenal ulcer, gastric ulcer, GERD
Proton Pump Inhibitors (PPIs):
H+/K+/ATP ase pump takes ____ out of cell and ____ into cell
This Proton pump is the final __________step in the production of gastric acid
Do PPIs inhibit basal and stimulated acid secretion?
H+ out of cell into stomach lumen and K+ into cell
COVERGENT
YES BASAL AND STIMULATED because they are INVERSE agonists
PPI final convergent step:
H+/K+ ATPase pump is located within the __________ site in resting parietal cells
Stimulated proton pumps relocate to the secretary canalicular ______ of the parietal cells
In the final step of acid production, the activated proton pump transport ____ from the cytoplasm inside the cell TO the gastric lumen in exchange for K+ ions from the gastric lumen INTO partietal cells
do PPIs bind reversibly or irreversibly to proton pump?
intraccellular
travel to membrane once stimulated
H+ from cytoplasm inside cell —> gastric lumen
K+ from gastric lumen —> into parietal cell
IRREVERSIBLY
PPIs:
what is true about the structure of ALL PPIs?
is there chirality?
what is true about omeprazole, lansoprozaole, pantoprazol, rabeprazole, and tenatoprazole?
sulfoxide group and benzimidazole
YES
they are all racemic mixures
Sulfoxide Chilrality:
A racemic PPI has (S) and (R) enantiomers. which is more active?
Consdider ________ as the substituent of lowest priority
Equivalent S and R
lone pair of electrons = substituent of lowest priority
MOA of PPIs via catalyzed generation of an ______ _________ ( )
PPIs get activated at the _______________ region of the partietal cell
what is the pka of benzimadazole?
active intermediate (sulfenamide)
PPI activation occurs at canalicular region
pka of benzimidazole = 6
Mechanism of action PPIs:
proton pump inhibitors are considers ________ bc/ they require activation by an ______ to an unstable reactive intermediate that will participate in COVALENT BOND FORMATION
after absorption the PPI diffuses into the parietal cells of the stomach wall and accumalates in the acid secreteor __________
Here, it will undergo ____-catalyzed rearangement into a tetracyclic _______
the activated form bind IRREVERSIBLEY to _____ residues (forming covalenr disulfide bond) in the ACTIVE SITE inactivating the enzyme
PPIs considered PRODRUGS bc/ require activation by ACID
accumulate in canacculi
in canaculli undergoes PROTON-catalyzed rerageent into SULFENAMIDE
To rpevent degredation of PPIS by acid in the gastric lumen, _____ dosages are formulated so that they will disolve only in the ____ pH of the ________
PPis are formulated as _____- coated granules in capsles or ___- coated tablets
oral dosage will only disolve in ALKALINE pH of intestine
enteric coated prevent from being disolved in stomach, and only released in intestine
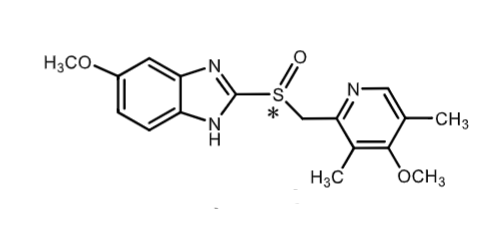
Omeprazole (Prozac):
racemic
what is the MOA?
what indications is it used for?
weak _____ base that gets protonated in parietal cell cannicula (prodrug)
how is it metabolized?
are its S and R enantiomers metabolized the same way?
PPI (irreversibly inhibit H/K ATPase)
GERD (20mg) and duodenal/gastric ulcers (40mg)
weak imidazole base
CYP2C19
no S and R metabolized differentially
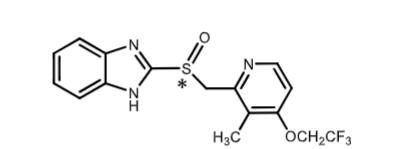
Lansoprazole:
racemic
what is its MOA?
what is it used to treat?
it is metabolized to sulfone derivative and hydroxymethyls (which enzyme does each)?
what is its oral bioavailability?
what is its main mode of metabolism?
PPI
GERD and duodenal/gastric ulcers
2C19 (hydroxymethyl) and 3A4 (CYP3A4)
80% oral bioavailability
elimination through BILE
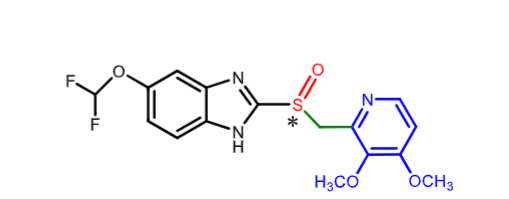
Pantoprazole:
racemic
what is it indicated for?
how is it metabolized?
how is it eliminated?
long term treatment of hypersecretory disordrs including ZOLLINGER ELLISON syndrome
CYP2C19 (demethylation) and 3A4 (sulfone)
kidney
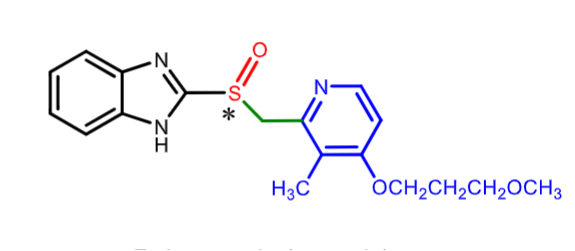
Rabeprozoole:
racemic
MOA ?
what is it used for?
what is its oral bioavailability?
how is it metabolized
which enzyme’s genetic polymorphisms results in slower metabolism in some populations causing HIGHER plasma levels of drugs
PPI
duodenal ulcers/ GERD/ hypersecretory conditions
52% oral bioavailability
CYP3A4 and CYP2C19 (can lead to toxic levels)
Metabolism of PPIs:
where is the major site of metabolism?
which PPI is less metabolized by CYPS than other PPIs?
differential metabolism is seen for the R and S enantiomers of _______
________ (Nexium) is the S isomer of ________ (greater bioavailability) in high CYP2C19 metabolizers and less interinndicidual variations among those who have varient alleles of CYP2C19
liver
rabeprazole
omeprazole
esomeprazole = S isomer of omeprazole metabolized by CYP2C19
hydroxylation and o-demethylation metabolism of PPIs is done by ______ and sulfoxidation is done by _______
CYP 2C19 = o-demethylation and hydroxylation
CYP3A4 = sulfoxidation
Generation 2 PPIs:
what makes gen2 PPIs different from gen1?
what are the gen 2 PPIs?
gen 1 = racemic
gen 2 = ONE enantiomer
gen 2 =
esomeprazole (S enantiomer of omeprazole)
dexlansoprazole (R enantiomer of lansoprazole)
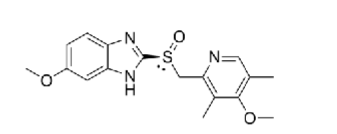
Esomeprazole:
2nd gen PPI (__ enantiomer of ______)
what is the advantage of the single enantiomer vs racemic mixture?
what is it used for?
S enantiomer of omeprazole
S enantiomer has HIGHER ORAL BIOAVALABILITY and LESS VARIABILITY OF CYP2C19 METABOLISM
GERD, hypersecretory conditions
Dexlansoprazole:
2nd gen PPI (___ enantiomer of ____)
what is advantage of this isomer over racemic mixture?
Indications?
R enantiomer of lansoprazole
HIGHER ORAL BIOAVAILABILITY and LESS INTER- INDIVIDUAL VARIATIONS
indications: GERD and EROSIVE ESOPHAGITIS
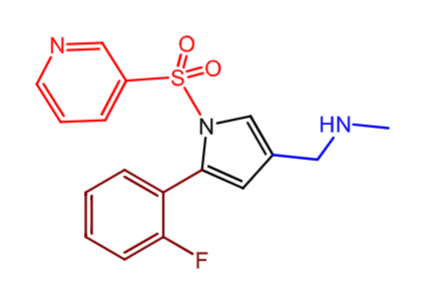
Vonoprazan (Voquenza):
what is the moa?
vonoprazan can inhibit both ______ and ________ gastric acid secretion like traditional PPIs
_____ agonist/antagonist
is it reversible or irriversible?
does it require activation by acid in parietal cell canicula?
moa: noncovalent PPI (compete with binidng of K+ instead of blocking active site of pump) - PCAB (potassium competitive acid blocker)
inhibit K+ baseline AND at gastric acid secretion
inverse agonist of the pump
REVERSIVBLE
doesn’t require activation by acid
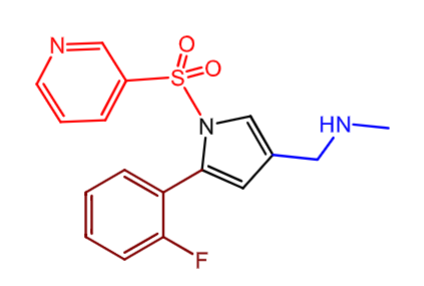
Vonoprazan (Voquenza):
what is it used for?
what are some warnings?
how is it metabolized? are these metabolites active or inactive/
indication:
GERD (erosive or non erosive)
H. Pylori eraddication WITH antibiotics
warning and precautions:
renal- actute tubulointerstitial nephritis (TIN)
clostridiodes dificil infection
osteoporosis/ bone fracture like other PPIs
Metabolism:
inactive metabolites
CYPP3A4/5, 2C19, 2C9, 2D6, 2B6