Nuclear chem
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
stability of nucleus depends on
Too many neutrons, protons or both
Ideal: 1:1
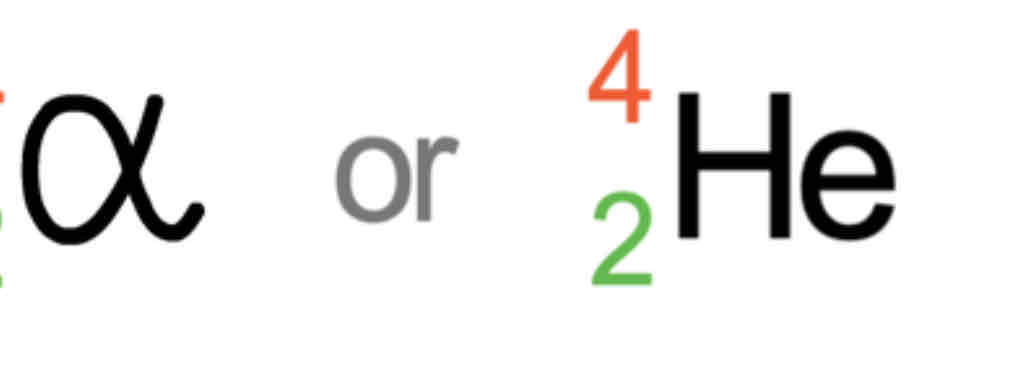
Alpha
Particle
Emitted
2 protons and 2 neutrons (charge of 2+)
Shielding : paper or layer of skin cells
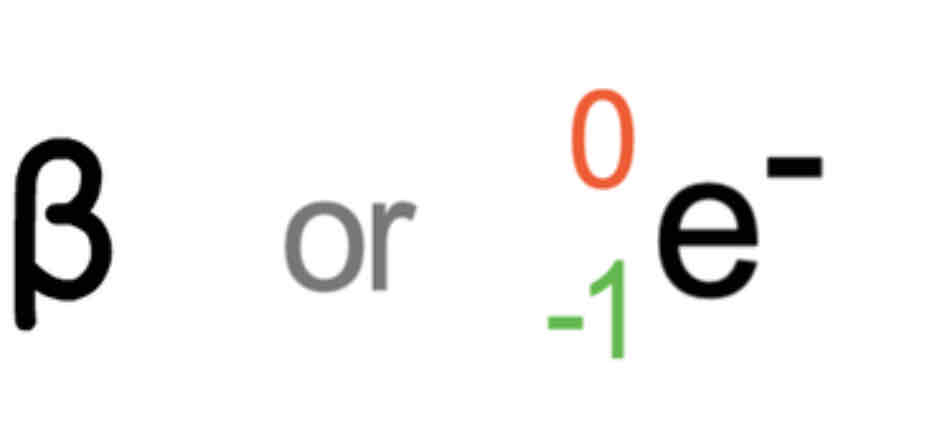
Beta
Particle
Emitted
Fast moving electron (-1 charge) NEGATIVE
Formed by decomposition of a neutron
shielding: aluminum foil or wood
Positron
Antimatter of an electron
Particle
Emitted
Positively charged electron (POSITIVE)
Formed by decomposition of a proton
shielding: aluminum foil or wood

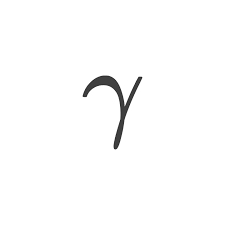
Gamma
Electromagnetic wave
Emitted
No mass or charge
Similar to x-rays
Pb(lead) or concrete
Electron capture
electron capture and gamma ray emitted
Wave
Shielding : pb(lead) and concrete

Half life
Time it takes for half of a radioisotope sample to decay
100% —> 50% —> 25%
Fission
1st step in chain reaction
Spitting nucleus U (uranium) or Pu (plutonium)
Nuclear waste
Used in nuclear reactions
Controlled fission: coolant (slows neutrons) and control rods (absorbs neutrons)
Requires neutron bombardment
Mass of product is slightly less than mass of reactant “missing mass”= energy
Fusion
Combining nuclei
H or He
Produces more energy
SUN
requires a high temp
Ions
Change in # of electrons
Cations: + loses electrons
Anions: - gains electrons
Isotopes
Change in # of neutrons
Changes mass number