orgo 1 sn2 sn1 e2 e1
1/68
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
69 Terms
the more substituted the carbocation, ___
the more stable the carbocation is
SN1 and E1 both have…
…a carbocation intermediate
Rank the subsitution of carboctaions from least to most stable
Methyl < Primary < secondary < Tertiary
What is more stable than a tertiary carbocation?
an allylic carbocation
What is more stable than an allylic carbocation?
a benzyllic carbocation
What should you look for when analyzing the carbon chain for an SN1 or E1 reaction?
look for a seconadry or tertiary leaving group. SN1 and E1 cannot occur with a primary leaving group.
What should you look for when analyzing the carbon chain for an SN2 reaction?
a minimally substituted leaving group. methyl or primary is best. also look for a small, less sterically hindered electrophile.
What should you look for when analyzing the carbon chain for an E2 reaction?
a primary, secondary, or tertiary leaving group. no methyls. it must a beta carbon with hydrogens.
What is the driving force for each reaction type?
SN1 and E1 - carbocation stability
SN2 - nothing specific
E2 - alkene stability
What are some general characteristics of SN2 and E2?
both bimolecular
strong nucleophile/base
“bully”
no carbocation intermediate
fast rxn
What are some general characteristics of SN1 and E1?
both unimolecular
weak nucleophile/base
“shy kid”
slow rxn
carbocation intermediate
How does charge relate to nucleophile and base strength?
A ngeative charge makes a strong nucleophile and a strong base.
How do periodic trends relate to nucleophile and base strength?
electronegativity increases to the top right of the periodic table. a less electronegative molecule is a better nucleophile.
nucleophilicity ___ down a column in the periodic table (only in polar protic)
increases
nucleophilicity ___ down a column in the periodic table (only in polar aprotic)
decreases
the more sterically hindered a molecule, the ___ it acts as a nucleophile
worse
How do you identify a polar protic solvent? what reactions do they favor?
a polar protic solvent has O or N attatched to a hydrogen. they favor SN1 or E1 reactions.
How do you identify a polar aprotic solvent? what reactions do they favor?
a polar aprotic solvent does not have O or N attatched to a hydrogen. they favor SN2 or E2 reactions.
How do you identify a nonpolar solvent? what reactions do they favor?
a nonpolar solvent will typically be a hydrocarbon. they favor Sn2 or E2
E1 reaction steps
leaving group leaves, forming a carbocation intermediate
base abstracts a proton from an adjacent carbon, forming a double bond and an alkene product
E2 reaction steps
base attacks beta hydrogen
hydrogen’s electrons collapse into pi bond
LG leaves
concerted
Sn1 reaction steps
LG leaves spontaneously
nucleophile attacks carbocation
Sn2 reaction steps
nucleophile attacks carbon holding LG
LG leaves
E1cB reaction steps
Deprotonation of the substrate to give an anion (its conjugate base)
Loss of a leaving group to give a new C-C pi bond.
what makes a good leaving group?
a weak base, or the conjugate base of a strong acid.
why are larger atoms better leaving groups?
they are able to spread out a negative charge better, making them more stable.
why is oxygen usually a bad leaving group?
it is a small atom and is rather reactive.
how can you make oxygen a better leaving group?
use an acid catalyst to form oxonium and then H2O
use tosylate or mesylate
why do mesylate and tosylate improve oxygen’s leaving group ability?
they introduce resonance, which stabilizes the charge
What does SN1 stand for?
substitution nucleophilic unimolecular
what is the slow step/rate determining step in an SN1 reaction?
carbocation formation
what is the rate equation for sn1?
rate = k[electrophile]
what is it called when the solvent also acts as the nucleophile?
solvolysis
Do substitution reactions deal with a base or a nucleophile?
a nucleophile
what does SN2 stand for?
substitution nucleophilic bimolecular
what is the rate equation for SN2?
rate = [Electrophile][Nu]
What is important to remember about stereochemistry in SN2?
The nature of backside attack means the stereochemistry will be inverted for the product.
How do you initatiate using an acid catalyst to make OH a good leaving group (SN2)?
lone pairs from oxygen will reach out to neighboring hydrogen to form a bond.
How do you determine when something is a good base vs a good nucleophile?
Charge: something negatively charged will be both a good base and a good nucleophile
Steric Hindrance: more sterically hindered molecules will be worse nucleophiles
Solvent effects: protic solvents can surround and weaken nucleophiles
when is something a good base but a bad nucleophile?
when it is a very large/sterically hindered molecule
when is something a good nucleophile but a bad base?
when it is large and polarizable, or when it is stabilized by resonance
What does E1 stand for?
Beta-Elimination Unimolecular
What is the slow step/RDS in E1
carbocation formation
what is the rate equation for E1
rate=k[electrophile]
what is one hint that a reaction will favor elimination?
the presence of heat
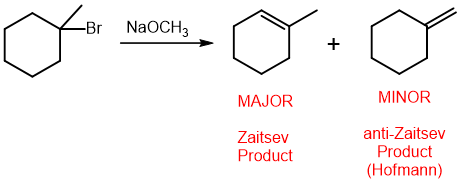
What is zaitzev’s rule?
an elimination reaction will form the more substituted alkene as the major product
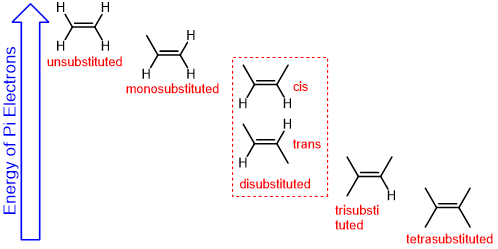
rank alkene substitution from least to most stable
unsubstituted < monosubstituted < disubstituted < trisubstituted < tetrasubstituted
What is a beta hydrogen?
a hydrogen one bond carbon away from the carbon holding the LG
Is the major E2 product E or Z?
E
what is something to remember about E1 rxns?
check for carbocation rearrangements
what does E2 stand for?
beta-elimination bimolecular
what is the rate for E2?
rate = k [base][electrophile]
In E2, the leaving group has to be ___ with a beta hydrogen
antiperiplanar
what is antiperiplanar?
on the same plane but anti to each other
True or false: the E2 reaction is concerted
true
What should you check for in an e2 reaction?
check to make sure that the leaving group and the beta hydrogens are antiperiplanar.
What do you do if the leaving group and beta hydrogen are not antiperiplanar in an E2 reaction?
rotate the newman projection and redraw the substrate
when you have an E2 reaction with a ring, what should you check?
turn it into a chair and make sure the H and LG are antiperiplanar
for the LG and H to be antiperiplanar, they must both be…
…axial
when making a chair for an E2 rxn that is a ring system, how should you number?
make the leaving group one so it will be axial
When an E2 reaction occurs with a very large base, what will happen?
the anti-zaitzev product, or the less substituted alkene, will be formed because the base will be too large to fit into the more substituted position
if you have a product with a pi bond, what can be said about the mechanism used to form it?
It was an elimination reaction.
what should you think when you see H2SO4 on top of the reaction arrow?
it is being used as an acid catalyst. it will dissociate to form an H+ ion.
the step with the higher activation energy is…
… the slow step
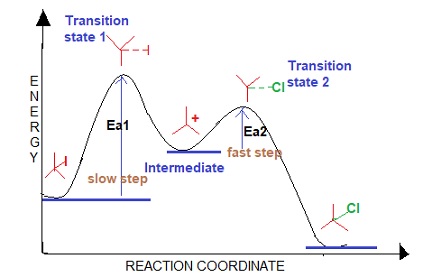
describe the SN1 energy diagram
two transition states
one intermediate
product is lower in energy than reactant
second transition state is lower in energy than first
slow step first
s
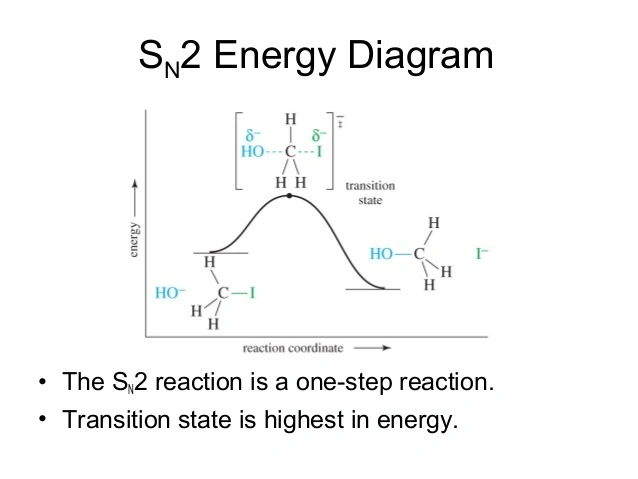
describe the SN2 energy diagram
one transition state
no intermediate
product is lower in energy than reactant
no slow or fast step, concerted
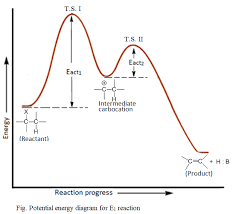
describe the E1 energy diagram
two transition states
one intermediate
product is lower in energy than reactant
second transition state lower in energy than first
rate determining step is first
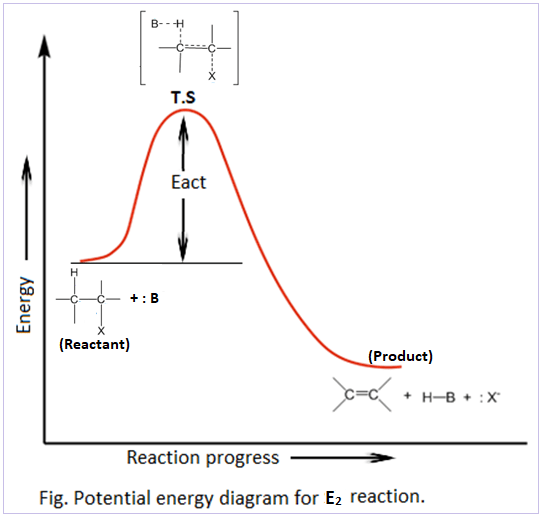
describe the E2 energy diagram
one transition state
no intermediate
product is lower in energy than reactant
no slow step, concerted
e
__ alkenes are major products in elimination reactions
trans/E