Unit Two Exam
1/95
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
96 Terms
When we say that an enzyme is a catalysts, what do we mean?
They accelerate reactions as much as 1020 times faster than the uncatalyzed reaction.
When we say that an enzyme is specific, what do we mean?
Typically very selective in substrate scope and catalyzed reactions. Usually stereo/regioselective.
When we say that an enzyme is a regulated, what do we mean?
Many mechanisms of regulation, such as controlling amount of enzyme in the cell or intermolecular interactions with regulators or activators.
How much can an enzyme accelerate a reaction?
By orders of magnitude.
Where do enzymes typically function?
In aqueous solutions under mild conditions (temperature, neutral pH, standard pressure) compared to synthetic routes to the same chemistry.
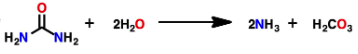
What makes the function of urease a good example of catalyst function of an enzyme?
Urease catalyzes the hydrolysis of urea into ammonia and carbon dioxide. Without the enzyme the reaction occurs once per a century, but with the enzyme there are about 30,000 reactions per second.
How does an enzyme interaction with a substrate occur?
Through a molecular recognition based on structural complementarity. The enzyme binds to the substrate at the active site, forming an enzyme-substrate complex.
What is the definition of an active site?
The site of substrate binding and catalysis.
What is an example of another important site in a protein other than the active site where catalysis occurs?
Allosteric site
What is the typical yield of an enzyme?
Over 95%
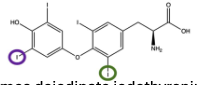
Why is thyroid hormone activation/degradation a good example of the specificity of an enzyme?
Thyroid hormone has four iodine groups attached to the molecule. In order to go from inactivated T4-Thyroid hormone to T3-Thyroid Hormone one of two iodine groups has to be removed by an enzyme. the enzyme can only remove a specific two iodine groups. Depending on which is removed the active form of the Thyroid hormone is chosen but the other iodine group being removed results in a maleformed hormone that is not functional. This highlights how enzymes selectively interact with substrates to produce specific products.
When we say that enzymes are usually stereoselective, what does this mean?
The preference of the substrate for a stereoisomer.
When we say that enzymes are usually stereospecific, what does this mean?
The preference to generate a specific product enantiomer.
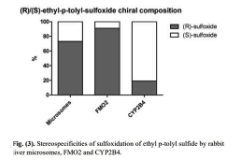
What does this figure about (R)/(S)-ethyl-p-tolyl-sulfoxide chiral composition saying?
That the microsomes are enriched mostly by the (R) enantiomer, which is formed by a specific enzyme, therefore that enzyme is the most present in the microsome.
Enzymes have multiple ways to regulate cells, what is an example of how an enzyme turns off transcription?
The blocking of the RNA Polymerase access to a gene.
Enzymes have multiple ways to regulate cells, what is an example of how an enzyme turns off translation?
The blocking of the ribosome’s access to a transcript.
Enzymes have multiple ways to regulate cells, what is an example of how an enzyme targeting an enzyme for degradation?
Ubiquitination
Enzymes have multiple ways to regulate cells, what is an example of how an enzyme undergoes reversible binding of an inhibitor or activator?
A complex protein structure means this binding can happen FAR AWAY from the active site and still affect catalysis, which is known as allostery.
Enzymes have multiple ways to regulate cells, what is an example of how an enzyme limiting access to substrates?
Product inhibition
What does this picture show?
That there are a lot of different metabolic pathways.
If an enzyme performs unnecessary metabolism, what does this cause?
wasted energy.
An example of an enzyme that is regulated is a lac operon, what is this example?
The protein that prevents the cell from making lactose catabolic genes if glucose is present or lactose is absent. When lactose (technically known as allolactose) is present, it binds to the repressor and allows transcription of those genes.
Does ∆G tell us anything about the rate of a reaction?
No, it only indicates the spontaneity of the reaction.
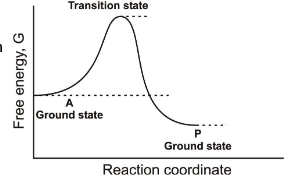
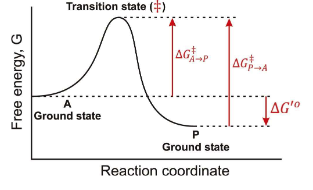
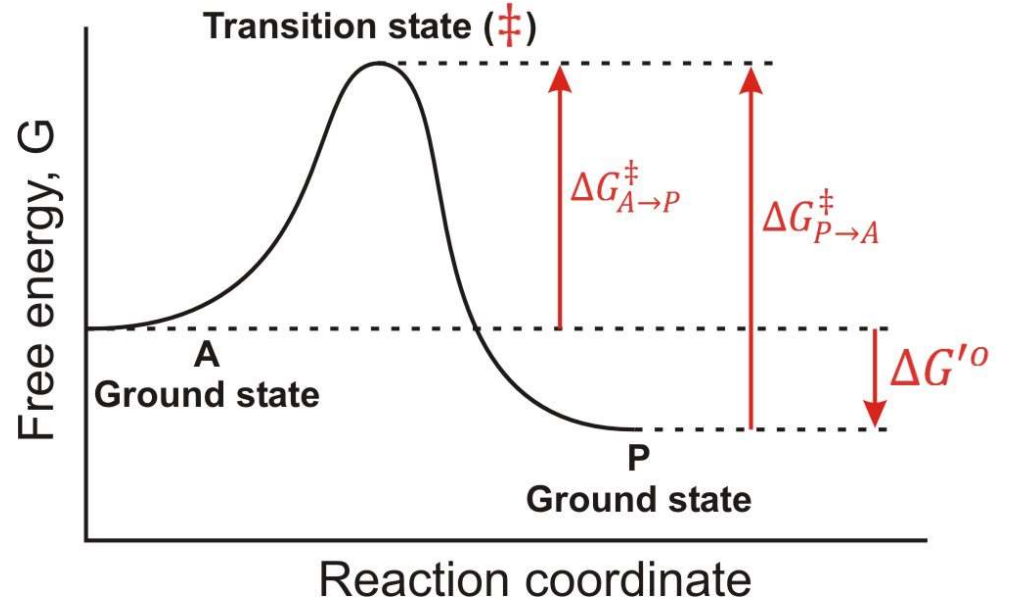
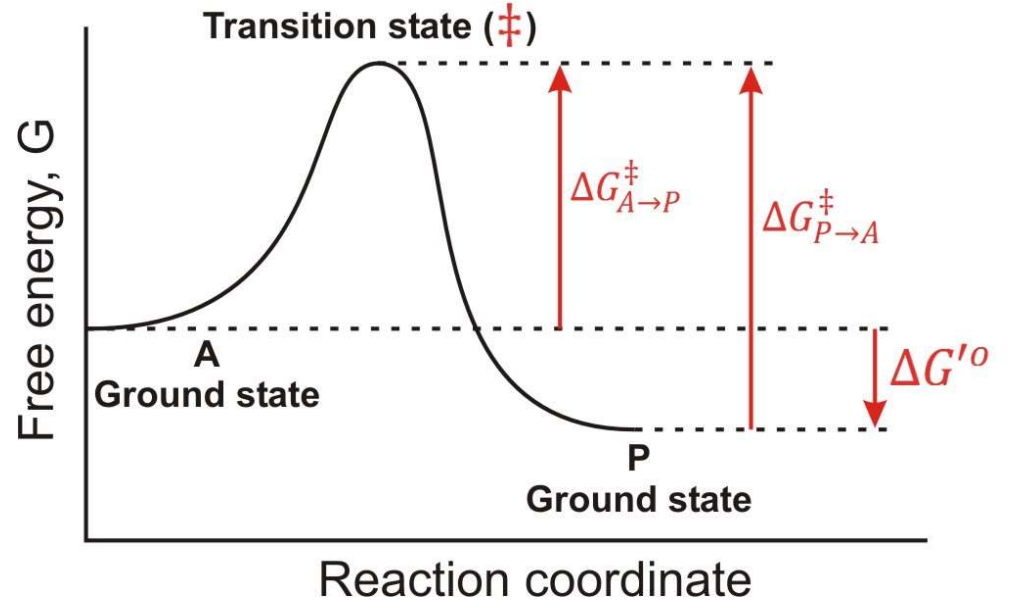
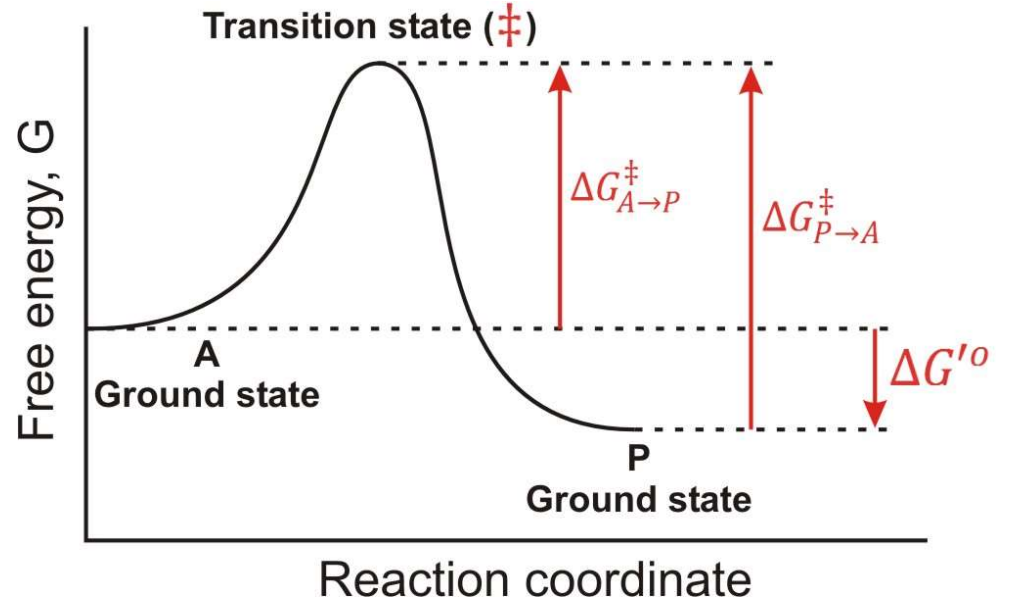
Between which two states is the overall ∆G for this reaction found?
A-Ground State and P-Ground State
What is Dr. Rush’s statement about thermodynamic kinetics?
“Enzyme catalyst change the rate or reactions but not thermodynamic position (∆G)”
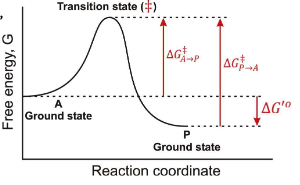
In a Free energy diagram, are there different barrier heights for forward vs reverse reactions?
Yes, they represent energy differences in activation energy.
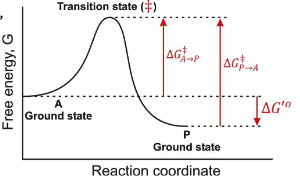
What are the other names for transition energy?
Activation energy and barrier height energy.
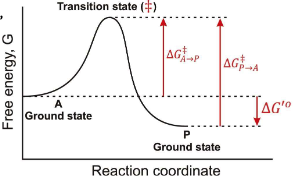
What is a transition state?
The transition state is a high-energy, unstable state during a chemical reaction where reactants are transformed into products, representing the peak energy point on the reaction pathway.
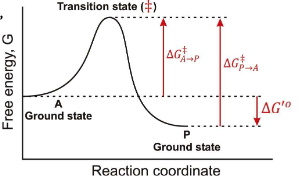
At the top of a Gibbs Free energy diagram, which is the top of the “hill”, what is the probability of decay to reacts? Products?
Statistically Equivalent states where reactants and products have equal likelihood of transition.
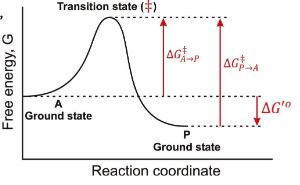
True or False: The Transition State is a reaction Intermediate?
False, it is a theoretical energetic description, not a stable chemical species.
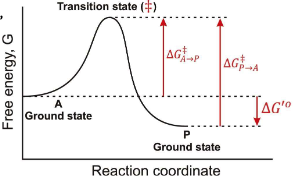
What does ∆G‡ mean?
The Activation Energy
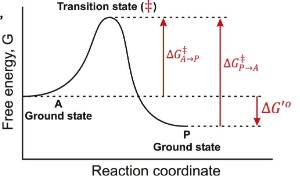
If you invert the direction of a reaction, what happens to ∆G?
It changes sign, so ∆G becomes negative if it was positive, and vice versa.
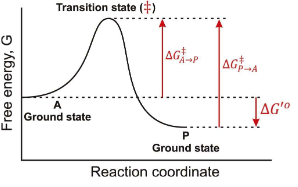
In the following graph if the overall ∆G for the reaction A → P is -12 kJ/mol. What is the ∆G for the reaction P → A?
12 kJ/mol
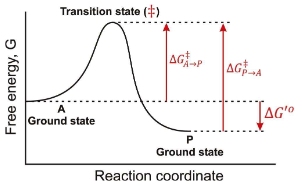
In the following graph if the overall ∆G for the reaction A → P is -12 kJ/mol. Estimate a value for ∆G‡ in the A → P direction?
34 kJ/mol
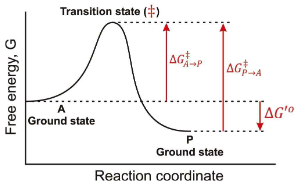
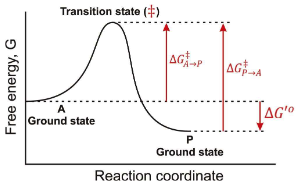
In the following graph if the overall ∆G for the reaction A → P is -12 kJ/mol. Estimate a value for ∆G‡ in the P → A direction?
46 kJ/mol
True or False: Once a system reaches a lower energy state, it is much more difficult to change.
True; lower energy states are more stable and require more energy to revert.
In teh respect of a Gibbs Free Energy, what does an enzyme do?
it works by lowering the transition state energy, and ∆G(A → P) is NOT CHANGED.
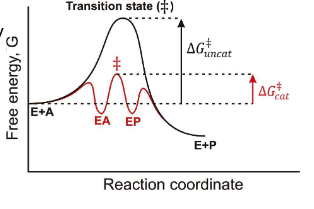
When looking at the following graph, what does EA mean?
the enzyme substrate complex
When looking at the following graph, what does EP mean?
the enzyme product complex
What affects the forward and reverse enzyme reactions?
The enzyme activity
What is the equation for differents states between the enzyme and substrate?
E + A ←→ EA ←→ EP ←→ E + Pq
What are the two ways to lower the energy barrier of a catalyzed reaction?
By stabilizing the transition state or destabilizing the initial
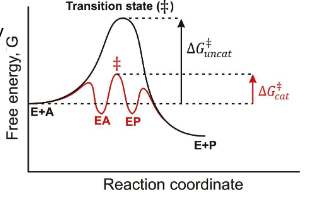
When we say, “Catalysis is reversible by definition” what do we mean?
The enzyme can catalyze the formation of the product and the enzyme can catalyze the undoing of the product.
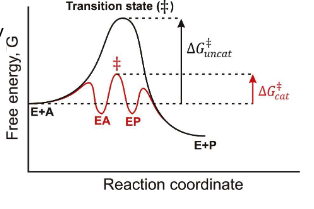
Is an enzyme reusuable?
Yes, enzymes are reusable because they are not consumed in the reaction.
For this reaction , A → P, the rate constant is connected to thermodynamics by?
k = Ae-∆G‡/RT
For a unimolecular 1st order reaction (A →k P), what is the rate given by?
v = k[A]
The problem with chemical rate laws for enzyme kinetic is that enzymes are complicated
.
What are some examples of analytical solutions that can be solved only for the simplest cases.
Models such as steady state and rapid equilibrium.
What effect will decreasing the ∆G‡ have on the value of k?
Increases, the rate constant
What effort will increasing temperature have on the value of k?
Increase, the rate constant
If the enzyme decreases ∆G‡ for the forward reaction by x kJ/mol, how will ∆G‡ for the reverse reaction change?
∆G‡reverse will decrease by the same x kJ/mol
Can ∆G‡ be negative?
No, it cannot be negative because the activation energy must always be a positive value for a reaction to proceed.
Can T be negative?
no, absolute scale (K)
How do we measure rates and analyze rates?
Through experimentation and observing changes in concentration of reactants or products over time.
Do you think the rates we measure in vitro are the exact same rates that enzymes are carrying out reactions in the cell? Why or why not?
No because the cell is such a complex combination o fbiological and environmental factors it is nearly impossible for the lab to perfectly mimic celllular conditions.
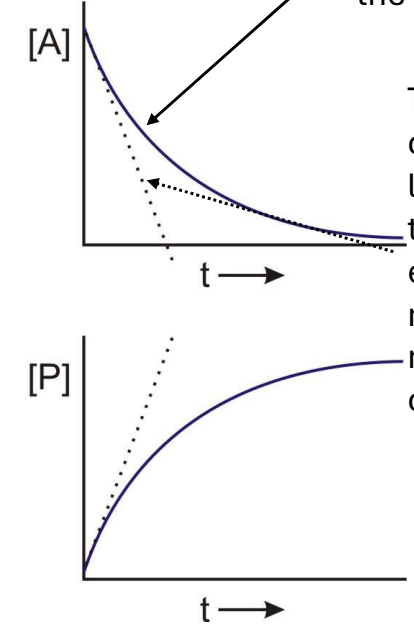
In the following graph what does the soldi line represent?
The actual [A] vs. time, described by the rate law.
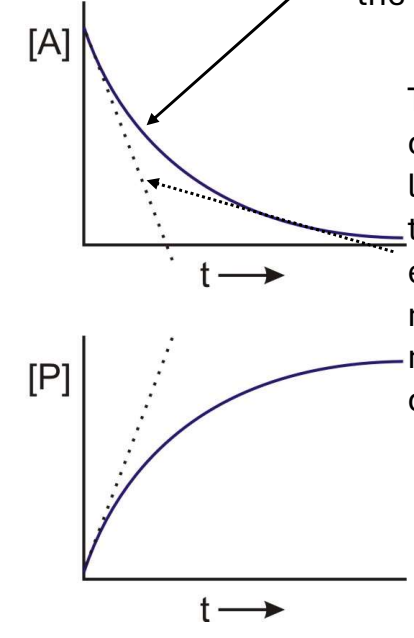
In the following graph what does the dashed line represent?
The initial velocity, a linear approximation of the reaction velocity at early time points in the reaction velocity at early time points.
For the reaction A →k P, what are the units of k?
s-1
For the reaction A +B →k P + Q, what is the order of this reaction? (overall and in each reactant)
Overall: second order
A: first order
B; first order
For the reaction A +B →k P + Q, what are the units of k?
s-1 M-1
Analytical solutions can be solved only for simplest cases, therefore what is used instead
Approximations
![<p>In the following plot which is of the Michaelis-Menton Theory, there are 4 phases of the plot of [P] vs time. What does A represent?</p>](https://knowt-user-attachments.s3.amazonaws.com/f911914b-9809-496c-a39c-8b9cba94bc48.png)
In the following plot which is of the Michaelis-Menton Theory, there are 4 phases of the plot of [P] vs time. What does A represent?
Pre-steady state (Burst phase); EA complex is formed, rapidly
![<p>In the following plot which is of the Michaelis-Menton Theory, there are 4 phases of the plot of [P] vs time. What does B represent?</p>](https://knowt-user-attachments.s3.amazonaws.com/7376e8d3-1dad-4f8a-bf6d-738a0c62710f.png)
In the following plot which is of the Michaelis-Menton Theory, there are 4 phases of the plot of [P] vs time. What does B represent?
Steady-State: [EA] is constant
![<p>In the following plot which is of the Michaelis-Menton Theory, there are 4 phases of the plot of [P] vs time. What does C represent?</p>](https://knowt-user-attachments.s3.amazonaws.com/acf6dce7-c81c-41b2-8147-7b2d3acd8918.png)
In the following plot which is of the Michaelis-Menton Theory, there are 4 phases of the plot of [P] vs time. What does C represent?
Product Inhibition; [P] cannot be ignored, k4 is happening.
![<p>In the following plot which is of the Michaelis-Menton Theory, there are 4 phases of the plot of [P] vs time. What does D represent?</p>](https://knowt-user-attachments.s3.amazonaws.com/4358463f-10f6-45bd-96f1-1f55ef0e0703.png)
In the following plot which is of the Michaelis-Menton Theory, there are 4 phases of the plot of [P] vs time. What does D represent?
Equilibrium; [A] and [P] are constant
Right before equilibrium the product builds up causing what?
The reverse reaction to occur.
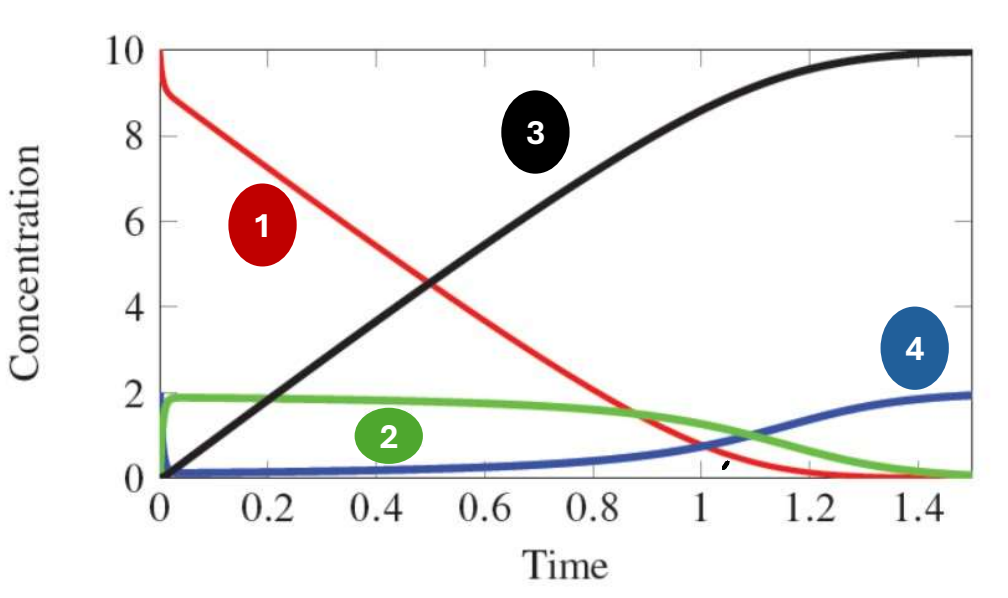
In the following graph of the following equation, which species does 1 represent?
E + A k2←→k1 EA k4←→k3 E + P
Substrate/Reactant
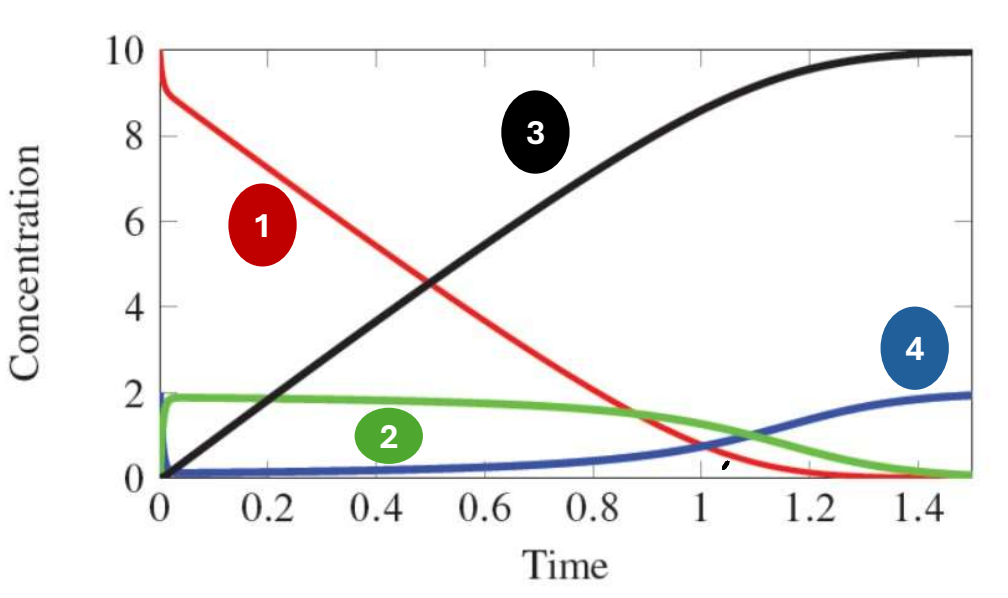
In the following graph of the following equation, which species does 2 represent?
E + A k2←→k1 EA k4←→k3 E + P
EA Complex
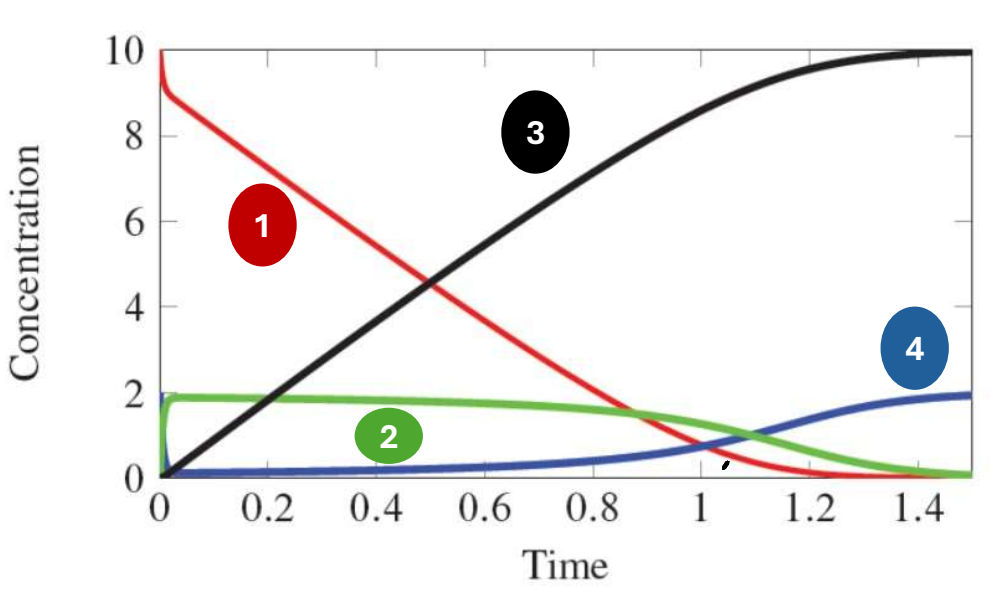
In the following graph of the following equation, which species does 3 represent?
E + A k2←→k1 EA k4←→k3 E + P
Product
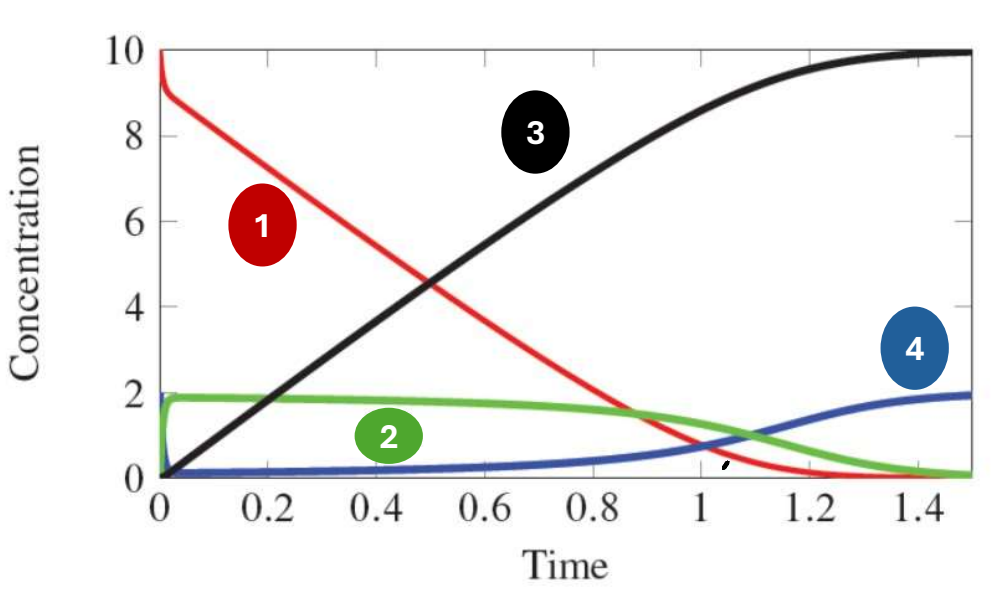
In the following graph of the following equation, which species does 4 represent?
E + A k2←→k1 EA k4←→k3 E + P
Enzyme
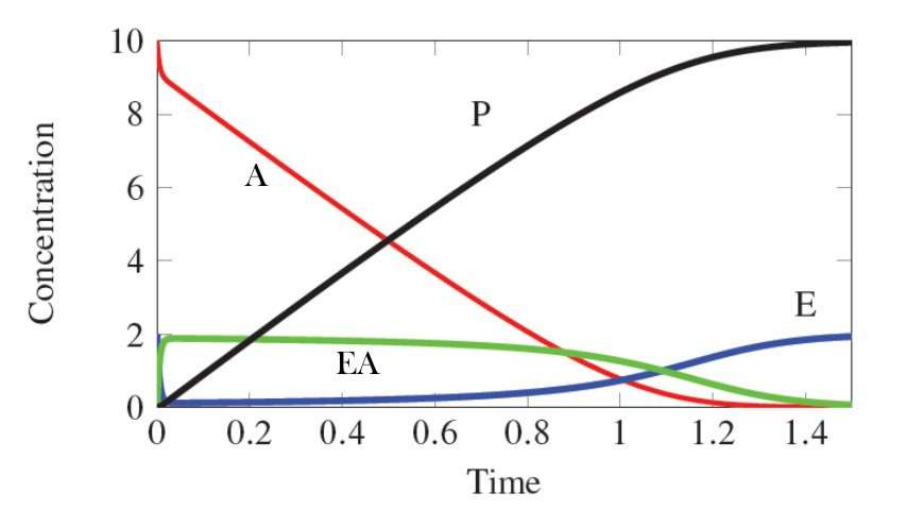
In the following graph what range of x-values corresponds to steady state?
0.01 - 0.2; [EA] is not changing, and the rate-measures is the linear range for [A] and [P].
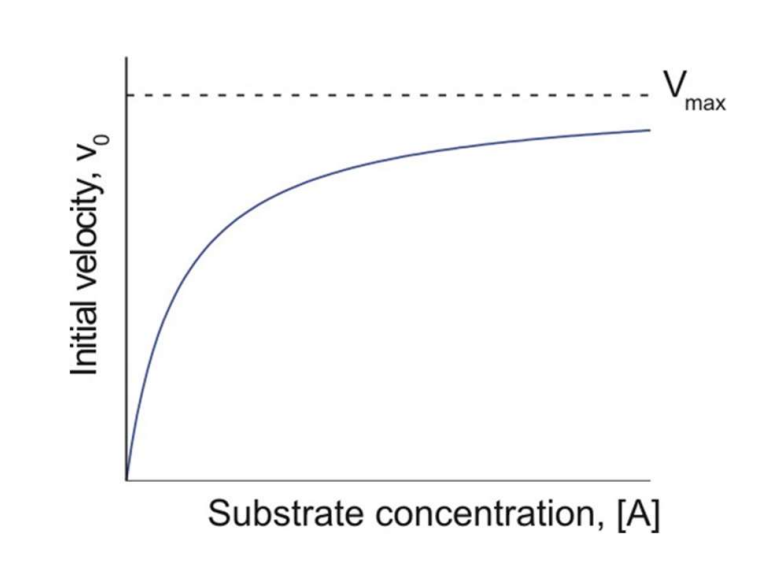
When looking at a Michaelis-Menten plot what is vmax?
The maximum velocity for a specific [enzyme].
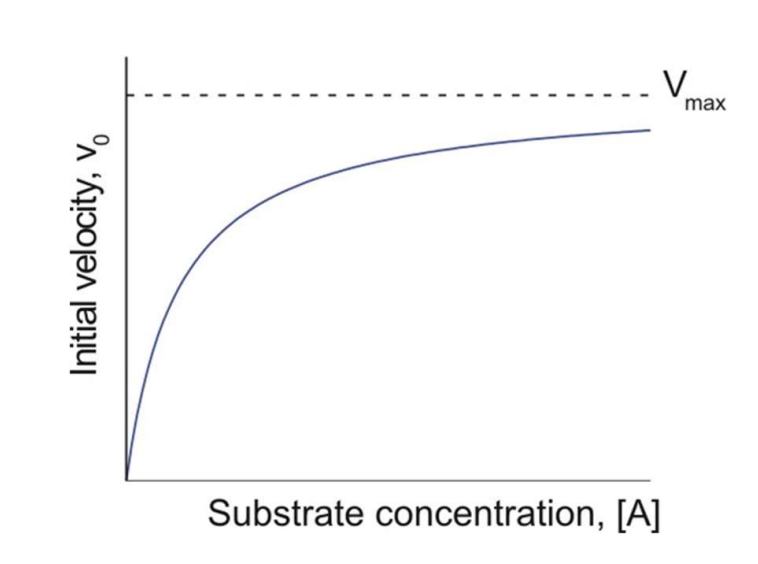
When looking at a Michaelis-Menten plot what is Km?
The [substrate] needed to reach half of the enzyme’s maximum velocity.
What is the first step in a steady state experiment?
To mix enzyme and substrates
What is the second step in a steady state experiment?
Measure [A] or [P] over time
What is the third step in a steady state experiment?
Repeat for variety of [A]
What is the fourth step in a steady state experiment?
Determine slope o fthe dotted line—this is intial velocity.
What are the assumptions of a steady state experiment?
[EA] is not changing making it an independent variable, and teh velocity is measure in the linear range (steady state) from [A] and [P].
At a low [A] what is the steady state kinetics doing
An almost linear increase of initial velocity with increasing [A]
At high [A]. what is the steady state kinetics doing?
initial velocity barely changes — this is maximum velocity for the reaction (vmax)
What is another name for Km?
Half saturating
What is Km dependent on?
all 3 rate constants; k1, k2, k3
What does a label like WT mean?
wild type, meaning the enzyme is as seen in the wild
What does a label like ∆EEVD mean?
It refers to a mutant with a deletion of a chain of amino acids in the enzyme.
What does a label like I637A mean?
It refers to a mutant where the isoleucine at position 637 is replaced by alanine.
Which variant has the highest vmax?
HSP72 WT
If you have two Km values and the enzyme with a lower vmax has a lower Km, what does it mean?
It indicates that the enzyme with the lower Km has a higher affinity for the substrate, allowing it to reach half-maximal velocity at lower substrate concentrations.
In the Michaelis-Menton Theory, why is k4 gone?
This is one of the requirements of steady state conditions where the experiments are done close to t=0 so that k4 is negligible.
What is the assumption about the analyte/substrate in Michaelis-Menton theory?
That under pseudo 1st order condtions (steady state), there is so much excess [A] compared to [E] that we jut assume the [A] does not change.
What is assumed about [EA] in Michaelis-Menton theory?
it does not change
What is the Michaelis-Menton theory?
The maximum rate of the reaction Vmax is reached when all active sites are occupied, which is ensured when [A] is much larger than Km (another requirement of steady state)
What does Vmax equal?
k3 [E0]
What is the Michaelis-Menton Equation?
v = Vmax ([A]/{[A] + Km})
At a low [A], Km » [A] therefore …
the fraction reduces to [A]/Km (linear dependence of [A])
At high [A], Km « [A], therefore …
the fraction goes to one and becomes Vmax (zero-order kinetics).
When comparing the functionality of an enzyme to degrade different substances, what type of Km do you want
Large Km