Untitled
1/16
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
Define Ionization Energy
The energy required to remove one mole of most loosely held electron from one mole of gaseous atoms, to produce one mole of gaseous ions, each with a charge of +1
What is important to remember about the atoms being ionized?
that state symbols are important, ionization energy only works in gas state
What is Ionization Energy measured in?
Measured in kJ/mol
What is the notation for Ionization?
Q (g) → Q+ (g) + e- (removal of first electron)
Q+ (g) → Q2+ (g) + e- (for second electron and so on)
What is the simplified explanation of Ionization Energy?
a measure of the energy needed to pull the electron away from the nucleus
What determines how easily an electron can be pulled away?
the attraction between the nucleus and that electron
What factors affect the attraction between electrons and the nucleus?
Charge of the Nucleus
Distance of Electrons from the Nucleus
Screening/shielding of inner electrons
If an orbital has paired electrons, those electrons have repulsion
Tell me more about how the Charge of the Nucleus affects the attraction between electrons and the nucleus?
more protons = more charge = stronger attraction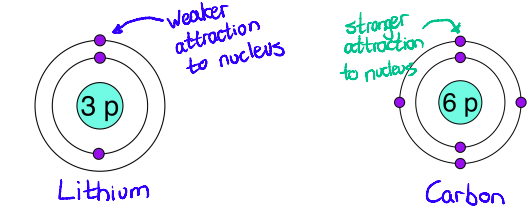
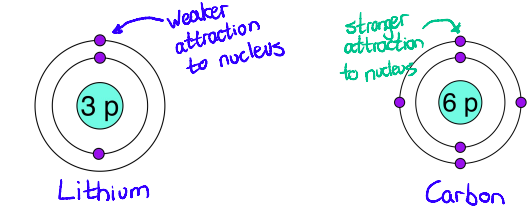
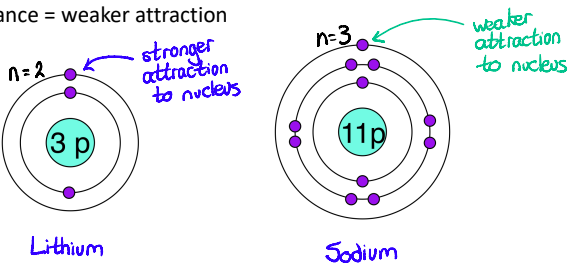
Tell me more about how the Distance of Electrons from the Nucleus affects the attraction between electrons and the nucleus?
larger distance = weaker attraction
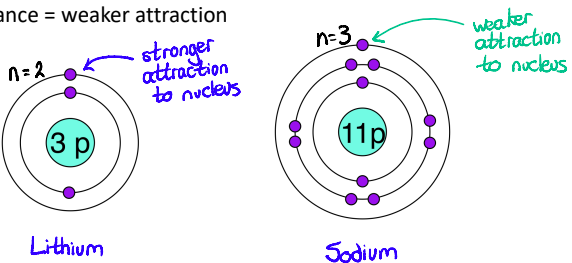
Tell me more about how the Screening/Shielding of inner electrons affects the attraction between electrons and the nucleus?
Inner electrons lessen the pull of the nucleus
Electrons in lower levels “shield” electrons in higher levels from the pull of the nucleus
e.g. Sodium electrons: 2, 8, 1, if the outer electron were to “look” down, it wouldn’t see the nucleus sharply because of the electrons below. It would only feel an effective nuclear charge of 1+

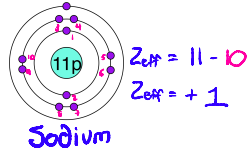
How does Effective Nuclear Charge work? How do you calculate it?
Each electron in a filled inner shell cancels 1 unit of nuclear charge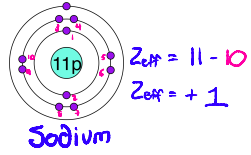
Tell me more about how the Repulsion affects the attraction between electrons and the nucleus?
If an orbital has paired electrons, those electrons have repulsion
A paired electron is easier to remove than a non-paired electron
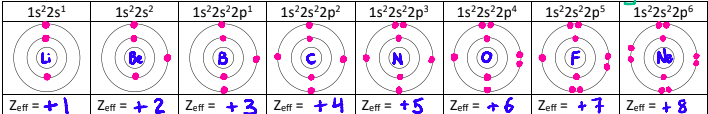
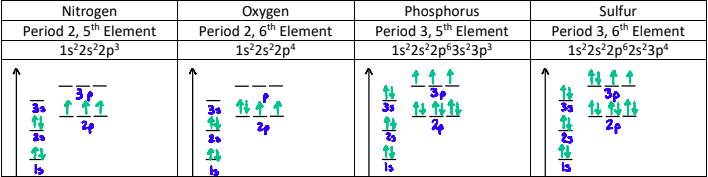
Why do the energies increase across a period?
Electrons being removed are all in the 2s or 2p orbital, are the same distance from the nucleus, and are shielded by the same number of electrons (the two electrons in n = 1).
However, the nuclear charge is increasing, and so is the effective nuclear charge since the number of shielding electrons stays at 2.
The greater nuclear charge pulls the electrons closer to the nucleus.
This trend is also seen in period 3. Although the effective nuclear charges are the same as in period 2, the increased distance from the nucleus lowers the ionization energy.
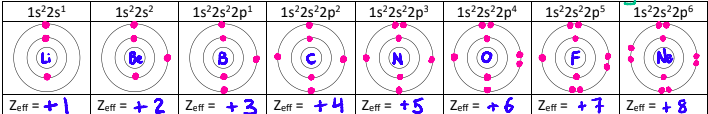
Why is there a dip between the 2nd and 3rd elements?
The outer electron in the p-orbital is easier to remove.
This is due to the p-orbital having more energy than the s orbital and being slightly farther from the nucleus. The p-orbital is also slightly screened by the s-orbital electrons.
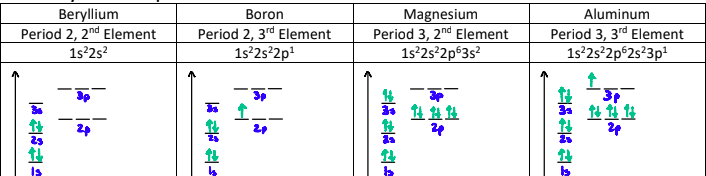
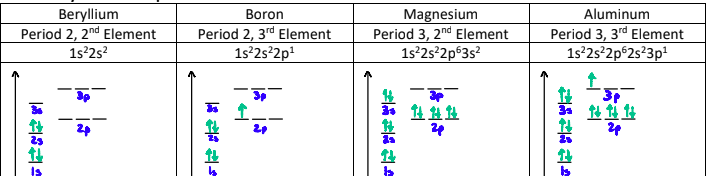
Why is there a dip between the 5th and 6th elements?
The p-orbitals in the 5th element are singly filled, whereas the p-orbitals on the 6th element are doubly filled.
When a p-orbital is doubly filled, there is repulsion between the two electrons sharing the orbital. This counteracts the effective nuclear charge.
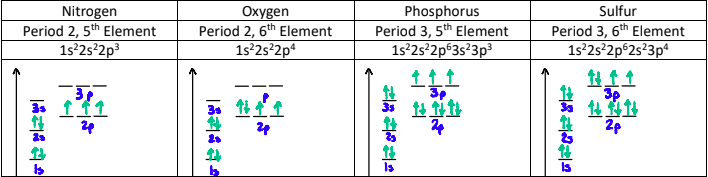
What is the General Pattern of Successive Ionization Energy?
When more electrons are removed, more energy is required to remove the next set of electrons.
What happens when removing Successive Electrons in the Same Energy Level?
When successive electrons in the same energy level are removed, more energy is needed for each electron removed.
As electrons are removed, there is a reduction in electron repulsion and increased attraction between the nucleus and electrons, which moves electrons closer to the nucleus
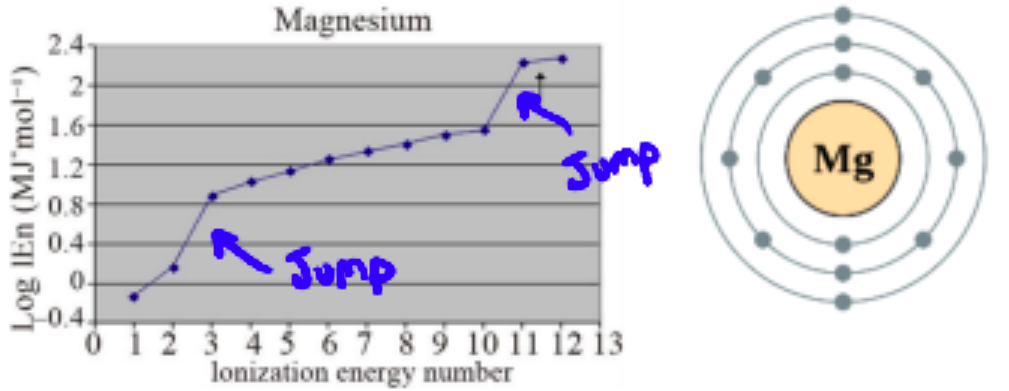
e.g. Magnesium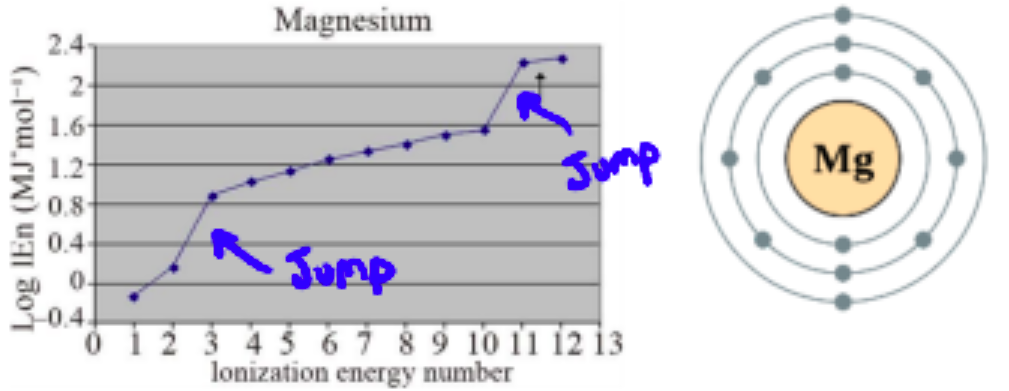
after valence electrons are removed, an inner energy level is reached and the Zeff increases, so there is a large jump in ionization energy. Since the jump happens after two ionizations, we know there must have been two valence electrons