7.5 Electrnegativity and Polarity
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
26 Terms
What is a Polar molecule?
Likes water (hydrophilic)
What is a nonpolar molecule?
Doesn’t like water (hydrophobic)
How is a 3D shape formed?
With the minimum repulsion between shared and unshared electrons.
Why is it a polar compound?
It is a polar compound because of partial charges (unequal sharing of electrons)

What is electronegativity?
The ability of an atom to attract/gain electrons in a chemical bond.
S- means?
Partially negative (More electronegativity likes to attract electrons more)
S+ means?
Partially positive (likes to attract electrons less).
EN___with___atomic number within a period.
Increases, increasing
EN___with___atomic number within a group.
Decreases, Increasing
Unequal sharing of electrons result in:
Polar covalent bond
Why are Nobel gases not listed when talking about EN?
Because they generally do not form compounds.
How do partial charges form?
1.When a polar bond forms, the shared electron pair or pairs are pulled toward one of the atoms.
2.The electrons spend more time around that atom than the other atom.
3.This results in partial charges at the ends of the bond.
True or false: Covalently bonded molecules are either polar or non-polar?
True
What does electronegativity difference (ΔEN) mean?
The difference between the electronegativity values of two bonded atoms.
Which type of bond has the smallest ΔEN?
Nonpolar covalent bond
Which type of bond has the largest ΔEN?
Ionic bond
Calculate the electronegativity difference:
H–Cl
H = 2.1
Cl = 3.0
0.9
Calculate the electronegativity difference:
C–H
C = 2.5
H = 2.1
0.4
Calculate the electronegativity difference:
O–H
O = 3.5
H = 2.1
1.4
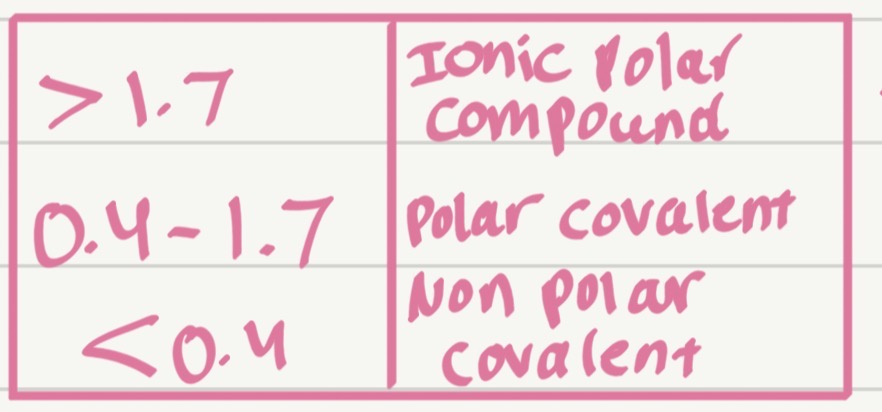
Classify the bond type:
H-H
H = 2.1
EN=0 Nonpolar covalent
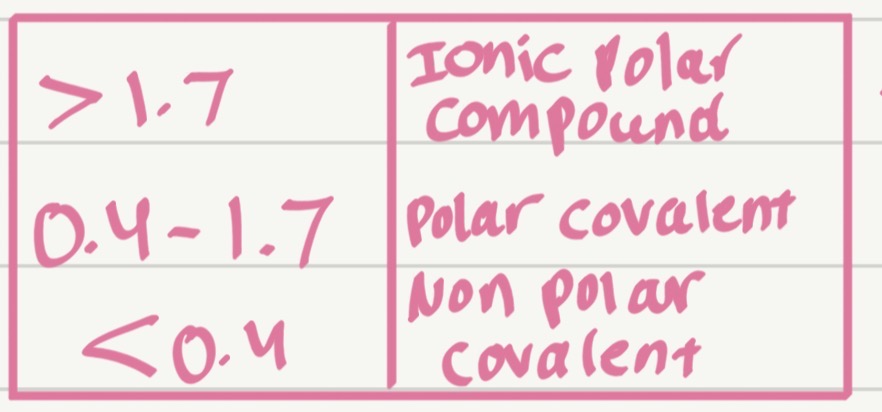
Classify the bond type:
Na–Cl
Na = 0.9
Cl = 3.0
EN=2.1 Ionic
Why does a larger ΔEN make a bond more polar?
Because of partial charges (unequal sharing of electrons), so electrons are pulled strongly toward one of the atoms.
Which atom becomes partially negative (δ−) in a polar bond?
The atom with the higher electronegativity
What happens to electrons in an ionic bond?
Electrons are transferred from one atom to another
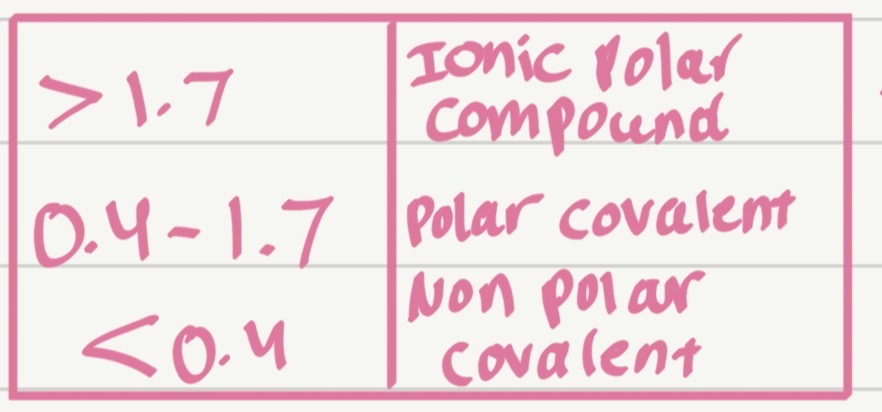
Arrange these bonds from least polar to most polar:
C–H, Na–Cl,O–H,
H = 2.1
C = 2.5
N = 3.0
O = 3.5,F = 4.0,Cl = 3.0,Na = 0.9
C-H<O-H<Na-Cl
A bond has a ΔEN of 1.2.
a) What type of bond is it?
b) Is it closer to ionic or covalent?
Polar covalent bond
Closer to ionic than nonpolar covalent