3.1H Ionization Energies of the Transition Metals
0.0(0)
0.0(0)
Card Sorting
1/3
Earn XP
Last updated 2:40 AM on 12/12/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
1
New cards
What did Structure 1.3 tell us about Ionization Energy?
Ionization energy is a measure of the energy needed to pull an electron away from the nucleus of an atom/ion
2
New cards
What determines how easily the electron can be pulled away from a nucleus?
The attraction between the nucleus and that electron
(Large IE = large attraction between electron and nucleus)
3
New cards
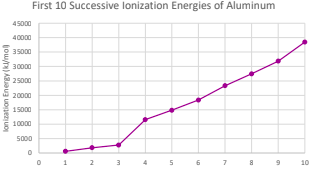
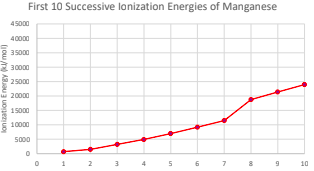
Why do transition metlas have variable oxidation states?
their successive ionization energies are close in value
4
New cards
What does the ionization energy of a normal metal look like?