NMSK - bones
1/154
Earn XP
Description and Tags
week 1 -
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
155 Terms
What do holes/depressions do/contain?
They normally contain and protect soft tissue structures such as tendons and nerves
What do these descriptive terms mean?
a) canal
b)fissure
c)foramen (foramina)
d)fossa (fossae)
e) meatus
f) notch
g)sulcus
h)groove
a) a bony tunnel
b) a narrow slit
c) a hole
d) a wide depression/hollow
e)a narrow passage
f)a large groove
g) a groove/furrow
h) an uncovered passage
What do these prefixes mean?
a)demi
b) epi
c) infra
d)inter
e) intra
f)sub
g) supra
a) half
b) above/upon
c)below
d) between
e) within
f) beneath
g) above
What do these descriptive terms mean?
a) anterior
b) distal
c)dorsal
d)external
e)inferior
f) internal
g)lateral
h)medial
i)posterior
j)proximal
k)superior
l)ventral/volar
a) nearer the front of the body
b) further away, the lower end of the body
c)the back surface of the body
d)outside
e)below
f)inside
g)away from the midline of the body
h)nearer the midline of the body
h)nearer the back of the body
i)closer to; the upper end of a bone
k) above
l) nearer the front of the body
j
Explain the histology of compact bone
very hard and strong
found mainly in the diaphysis of long bones, where a strong tubular structure is requires
forms the cortex of all bones
Consists of: Haversian systems (cylindrical structures)
A central Haversian canal - nerves, blood and lymphatic vessles
lamellae - concentric (like in a tree trunk) rings/layers of bone around the canal
lacunae - spaces b/w lamellae containing osteocytes
Canaliculi - channels carrying nutrient fluid that connect lacunae and communicate with the central canal.
Between the Haversian systems are Volkmann’s canals - they join them together and contain nerve, blood and lymphatic vessels
Explain the histology of spongy bone
also called cancellous bone
found where lightness, strength and increased SA are needed.
mainly in the end of long bones and middle of other bones
Similar structure to compact bone but no true Haversian systems
Rings of lamellae have an irregular lattice structure of thin columns of bone called trabeculae.
Trabeculae provide the internal support structure of bone and are aligned along the directional forces to provide tensile and compressive strength.
The spaces in the trabeculae help to reduce the overall weight of the bone
Contain bone marrow
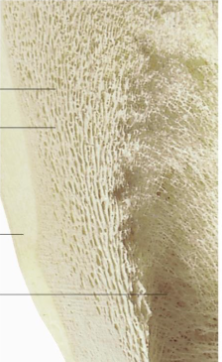
Label this image
Top - spongy bone
Trabeculae
Compact bone
Medullary cavity
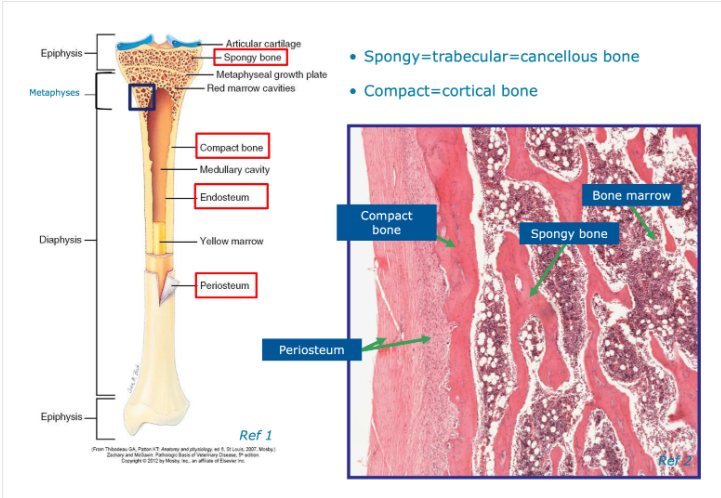
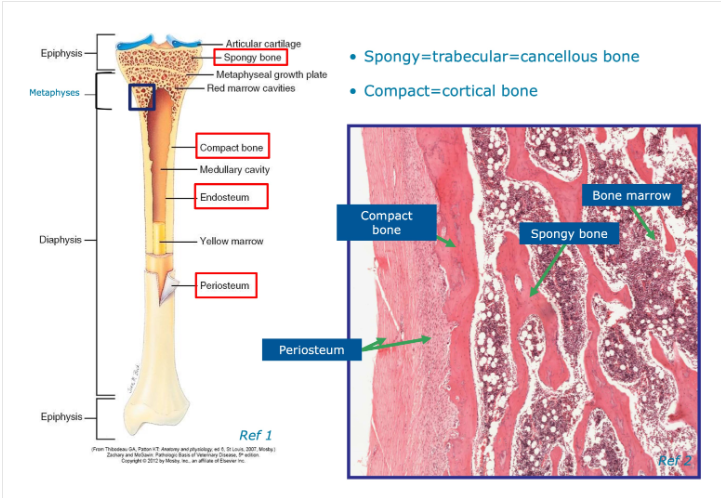
Where do we often find compact and cancellous bone on:
a)long bone
b)flat bone
a) Compact - forms outer circular surface
Cancellous - near the ends of long bones
b)Compact - forms the outer/inner flat surfaces
Cancellous - fills the narrow interior of flat bones
What type of tissue is bone?
A connective tissue in which mature bone cells are enclosed in an intercellular matrix of organic collagenous protein fibres called osteoid and a harder inorganic materialised component.
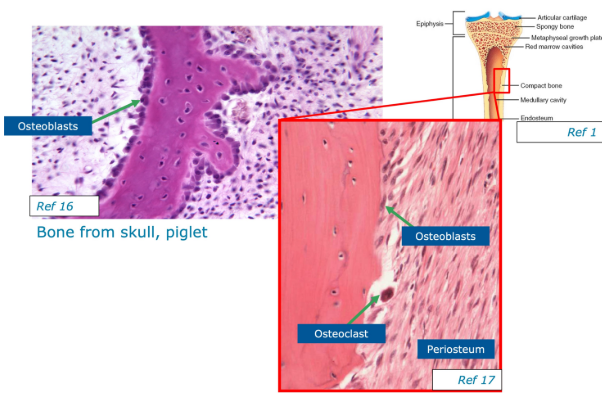
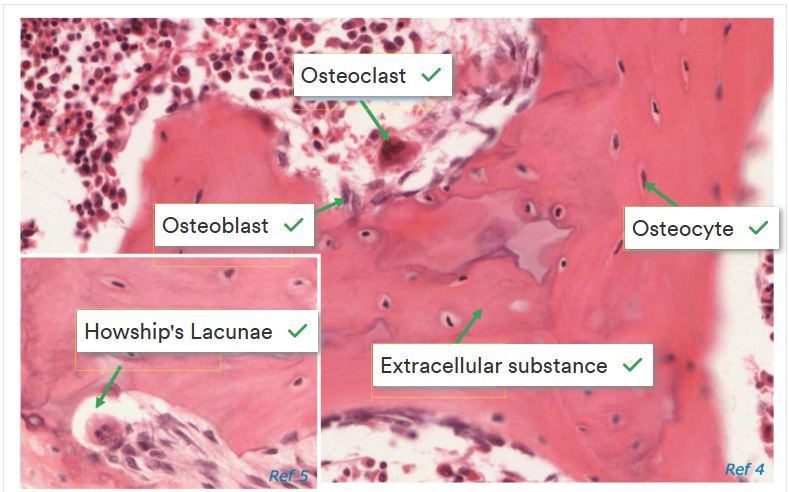
What are the 4 types of cells found in bone matrix?
1- Osteogenic cells - stem cells found in the inner aspect of the periosteum, the endosteum and in communicating channels between the units of bone. They divide to form osteoblasts
2- Osteoblasts - bone-building cells that secrete the osteoid, collagen and other components that build the organic matrix. They start the process of calcification for the inorganic mineral matrix in the spaces b/w collagen fibres
3- Osteocytes - mature bone cells that maintain the normal cellular activity of bone such as nutrient exchange. They are formed from osteoblasts when they become trapped in their matrix and no longer secrete osteoid.
4- Osteoclasts - bone-consuming cells. Large cells made of many monocytes that release strong enzymes to dissolve the bone matrix. Found mainly in the endosteum on the inside of the bone.
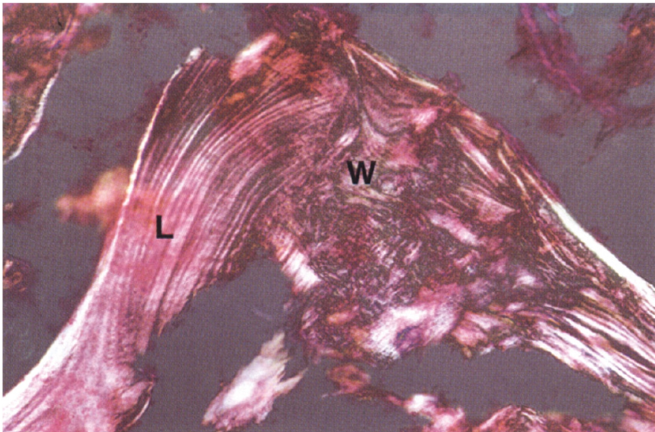
What is the difference between laminar and woven bone?
Laminar bone is mature bone
Woven bone is immature
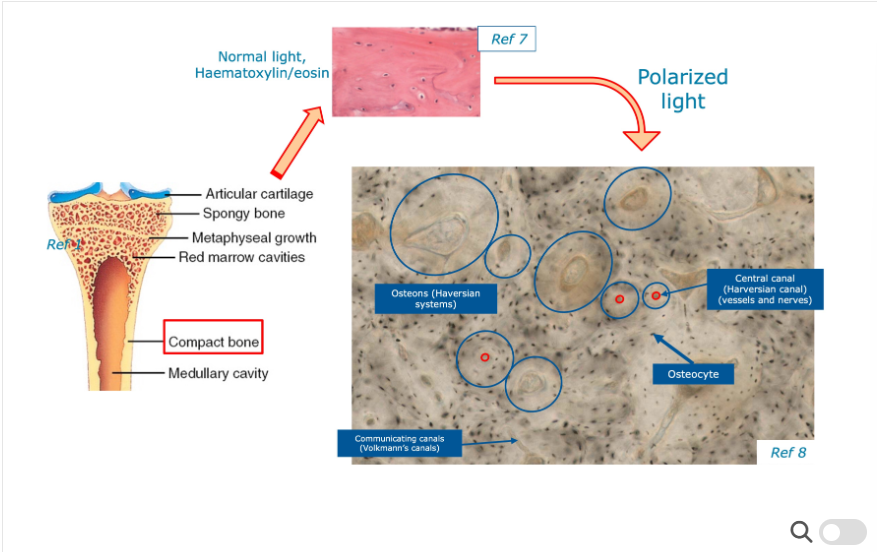
How do we observe bone in microscopy?
Need to stain using Haemotoxylin Eosin stain - appears pink under normal light conditions - stains a wide variety of cytoplasmic, nuclear and extracellular matrix features.
Then we apply polarised light to the tissue section which enables different structures to be seen slightly differently e.g. haversian canals you acan see osteocytes, volkmann’s canals and haversian systems.
What for and how do we use Schmorl’s stain
an alternative technique for examining bone, by sawing it into thin wafers using an abrasive surface - produces thin ‘ground’ sections.
It’s a dark stain which provides high contrast and enables open spaces to be seen in greater detail.
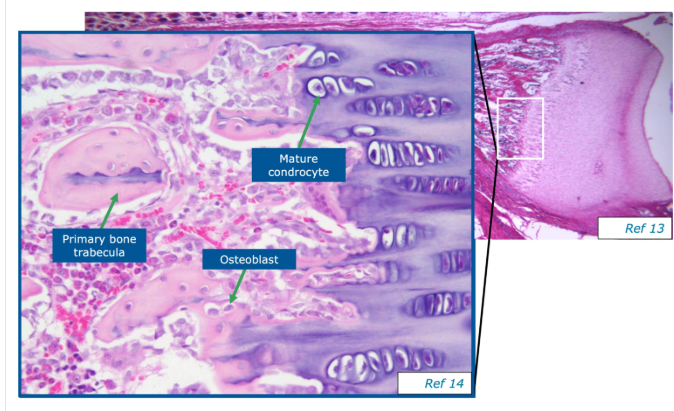
Explain Endochondral ossification
Begins with hyaline cartilage modle that is later replaced by bone. Occurs during most bone development at epiphyseal plates that enable long bones to grow in length.
what is osteogenesis and what are the two stages
A complex process consisting of cell migration, differentiation, extracellular deposition and mineralisation
The two stages are Intramembranous ossification (direct bone formation) and endochondral ossification (cartilage is a precursor to bone)
What is the primary centre of ossification
Appears in the middle of the diaphysis, osteoblasts appear and the bone matrix tissue is laid down.
Osteoclasts destroy/resorb bone and mould it to the required shape via remodelling. Responsible for forming the medullary canals/sinuses w/in bone.
Spreads from the middle of the diaphysis towards the epiphyseal plates at the end of the bone
Simultaneously bone is being built around the outside of the diaphysis and later forms the periosteum.
Bone width increases and is maintained by bone formation and turnover in sub-periosteal and endosteal bone surfaces
What are the secondary centres of ossification
Appear at the end of the bone and form epiphysis
Epiphysis is separated by a thin layer of cartilage (epiphyseal plate/physeal plate/physis) from the diaphysis
Typically forms the surface of an adjacent joint.
What is intramembranous ossification
occurs in a connective tissue membrane and begins during fetal development with the differentiation of mesenchymal cells into osteoblasts.
secretion of osteoids that undergo calcification to produce bone
Ossification spreads from the centre of the bone outwards
Forms most flat bones e.g. skull, mandible and clavicles.
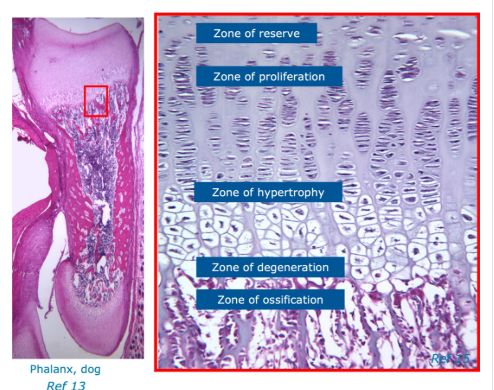
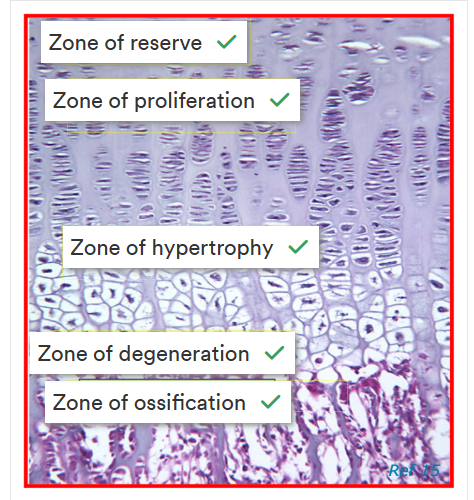
What are growth plates
a thin layer of cartilage b/w the epiphysis and metaphysis of long bones
Characterised by:
a resting/’reserve’ zone
a proliferation zone
hypertrophic zone
zone of degeneration
What is cartilage and what 3 types are there
Composed of cells. fibres and a highly-hydrated ground substance.
fibres provide tensile strength
proteoglycans in the ground substance make it resilient by trapping water (healthy cartilage should be ~70% water)
Avascular
3 types:
Hyaline Cartilage (type 2)
elastic cartilage (elastic fibres and type 2)
fibrocartilage (type 1 and 2)
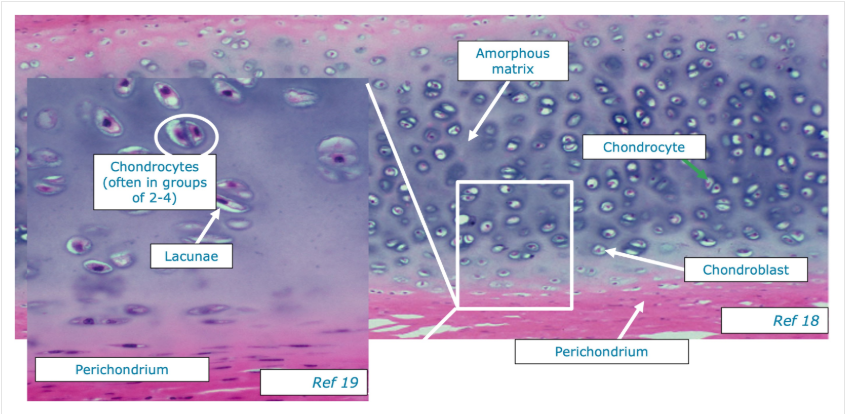
Describe the structure of hyaline cartilage
Lines the articular surfaces of synovial joints, acting as a self-lubricating shock absorber with low friction properties.
articular cartilage doesn’t have a perichondrium on either surface.
free (articular) space - exposed to synovial fluid within the joint
basal surface - in direct contact with underlying bone
Articular cartilage is the remnant of hyaline cartilage that formed the template for the developing bone.
limited to interstitial growth because of the absence of the perichondrium
No nerves/blood vessels.
Explain the structure of elastic cartilage
Similar to hyaline but the matrix also contains a dense network of branching elastic fibres - occurs where flexibility is required e.g. epiglottis, external ear and auditory tubes.
Perichondrium (blue)- a dense layer of irregular connective tissue that surrounds cartilage - 2 layers
Inner Chondrogenic layer (dark blue)- contains mesenchymal cells that differentiate into chondroblasts, initiate matrix production (elastin and type 2 collagen) and become immature chondrocytes.
Outer fibrous layer (blue) - fibroblasts produce type 1 collagen on the outer surface of the perichondrium
matrix - fibroblasts that produce type 1 collagen on the outer surface.
chondrocytes - cells within lacunae inside cartilage that occur singularly or in clusters (isogenous groups)
No blood or nerves
What is masson’s trichrome stain?
Stains connective tissue dark blue, smooth muscle and cytoplasm light pink and nuclei dark red-purple to black.
What does Aldehyde fuchsin stain?
elastic fibres pink-purple
Explain fibrocartilage
A mix of hyaline cartilage and regular connective tissue
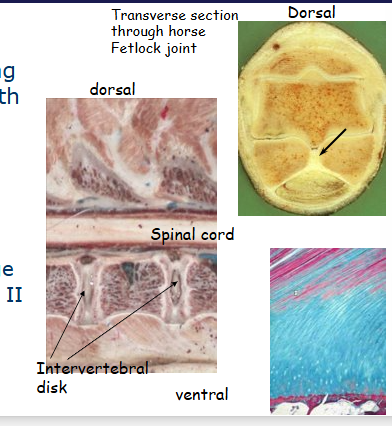
combines collagen fibre tensile strength with resistance compression of cartilage. Found where tendons attach to bones, minisci and intervertebral disks
Collagen fibres (type 1 and 2, stained pink/red)
fibroblasts - scattered w/in fibrous regions with elongated/flattened nuclei
chondrocytes - dispersed either singularly or in isogenous groups
matrix - much less surrounds each chondrocyte than in hyaline cartilage - type 2 collagen and proteoglycan ground substance. Basophilia is due to a high content of sulfated glycoaminoglycans (GAGs)
No perichondrium
Give a brief description of where synovial fluid should be found and what it should look like
Should be clear, pale straw colour/colourless and have the consistency of uncooked egg white (high viscosity due to hyaluronic acid)
Acellular liquid too (relatively)
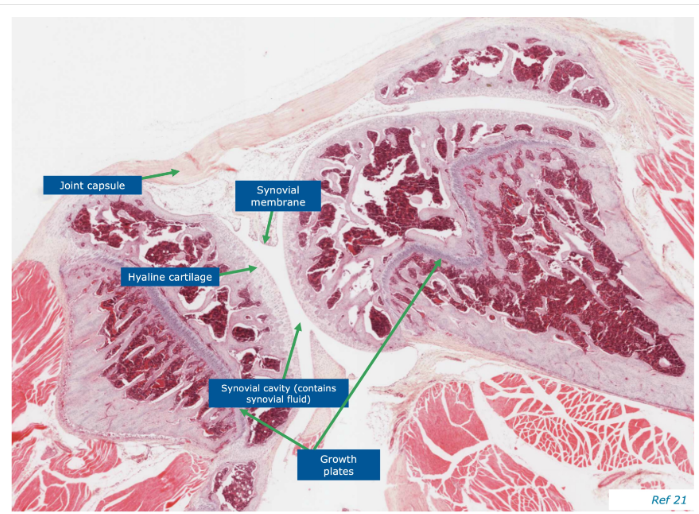
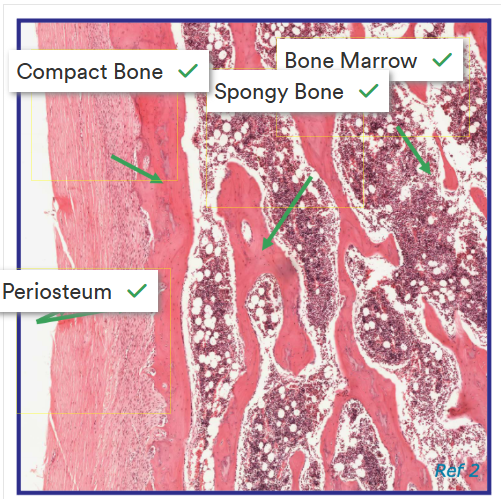
Image for identifying regions of a bone

Label this image correctly
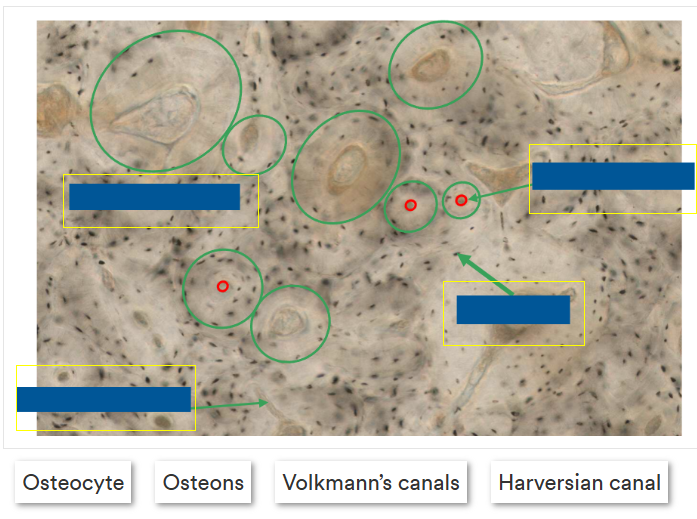
label this image correctly
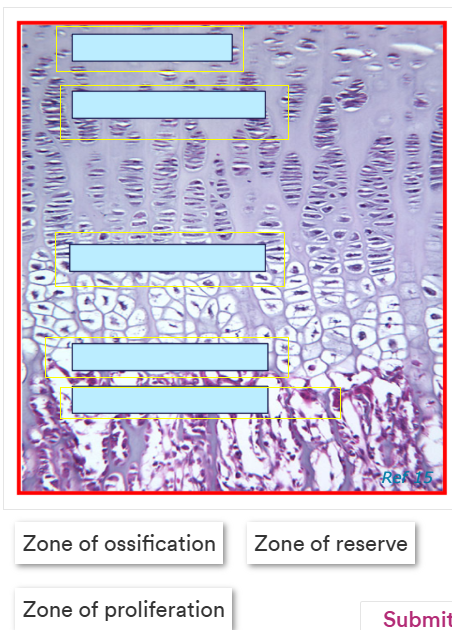
Label this image correctly.
Terms - zone of degeneration, zone of hypertrophy, zone of reserve, zone of proliferation and zone of ossification
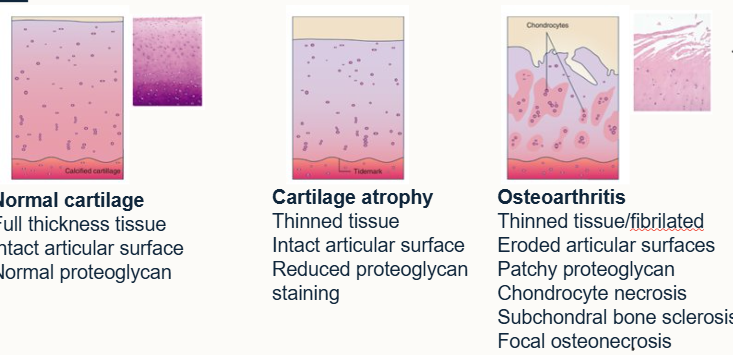
Define osteoarthritis
progressive deterioration of articular cartilage associates with changes in bone and soft tissue of the joint including loss of cartilage, subchondral bone sclerosis and marginal osteophyte formation
How do we grossly characterise osteoarthritis
Fibrillation, wear lines, erosion (partial/full thickness), peri-articular osteophytes, eburnation (cartilage has gone from spongy and complaint to rock hard)
why is cartilage hard to heal?
it’s avascular
How does osteoarthritis develop?
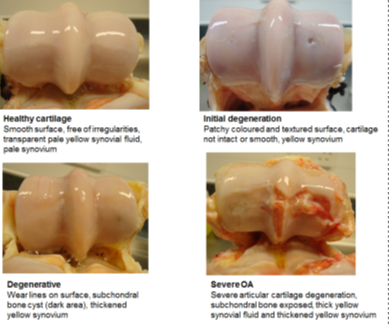
Where are some common sites of osteoarthritis in horses
distal articular joints (most common) - carpus, fetlock, distal interphalangeal joint (DIP J), hock, Proximal interphalangeal joint (PIP J)
where may we see OA in dogs?
Distal joints and hips (often develops early on and is very painful).
where are common places for OA for cats
distal joints, hips and all along the spide (in particular in the elderly cat).
May not compromise in its gait but the owner may say the cat is struggling to be incontinent or struggling to get into litter tray - due to the OA (can’t turn to clean rear end because of pain).
What happens in osteoarthritis.
It’s a complex condition that affects the whole joint and is now thought to involve cartilage, subchondral bone and the synovium. It’s likely they all have key roles in disease pathogenesis and an association with systemic inflammation could also be present.
Cartilage’s role:
the innate immune system is activated.
Chondrocytes express many toll-like receptors that are activated by damage-associated molecular patterns. They also express receptors that bind advanced glycation end products which accumulate with ageing tissue. This resultsin a phenotypic shift to catabolism (why OA is linked to age?)
These responses may also be a reflection of established cartilage degredation.
Chondrocytes could be first activated by inflam signals originating from other joint structures
Subchondral bone involvement:
Forms an interface b/w calcified cartilage below the tidemark and underlying trabecular bone.
Pronounced changes are seen in structure and composition in cortical plate and trabecular bone in OA
Features of endochondral ossification are reinitiated in OA and the tidemark advances with associated vascular penetration. Accompanied by osteophyte and subchondral cyst formation.
Highly innervated bone, likely contributes to the generation of pain in the disease
Synovium role:
synovitis is an early sign of OA
proliferation of synoviocytes and tissue hypertrophy are notable, with increased vascularity
Synoviocytes synthesise lubricants such as hyaluronic acid and lubricin - contribute to optimum joint function.
Reduced lubrication seen in OA patients.
Release inflam mediators and degradative enzymes.
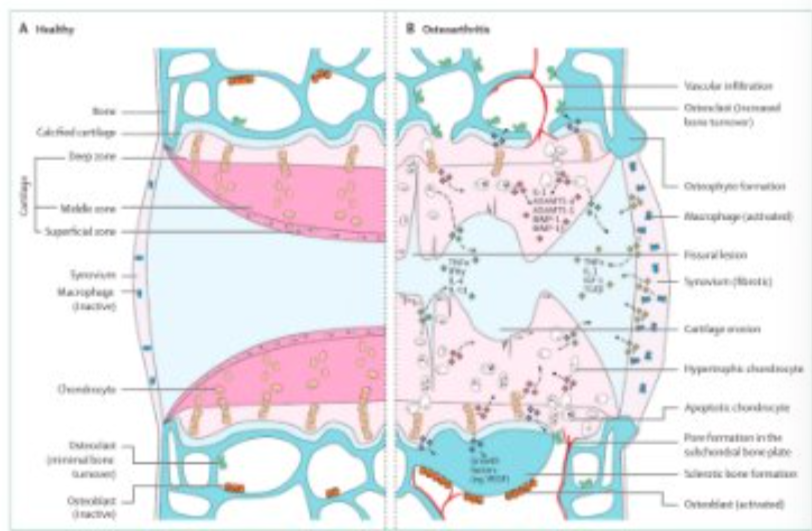
What differences can be seen in this image?
vascular infiltration seen in OA
larger synovium
activated macrophages
broken cartilage
thin, splits and cuts seen in the cartilage
lots of inflammation markers
How does OA cause pain?
Pain signals are sent from the joint to both the ‘higher’ brain function of the cerebral cortex, creating the feeling of pain itself.
Pain signals are also sent to the limbic system which results in behavioural changes - not wanting to jump, feeding changes, sleep changes, dream cycle changes, general attitude changes.
What does osteoarthritis look like histologically?
Cartilage becomes softer in compression and weaker in tension in OA
Proteoglycan loss
Altered collagen structure
Increased water content
What are osteophytes and why do they form?
In order to try and restore some strength and support to the bone, it may produce osteophytes (proliferation of a new cartilage and bone at joint edges).
what may osteophytes look like on a radiograph?
grainy, extra pieces of bone, give the impression that the bone isn’t smooth/has additional growth
what are some causes of osteophytes
ageing
mechanincal instability
secondary to synovitis
response to forces
tissue attachments
How can we diagnose OA?
TAKE A GOOD HISTORY
listen to what the owner says about the animal’s behaviour and how it’s changed
radiography and ultrasonography (used to confirm what we know, as the ultimate care plan won’t change that much)
Joint fluid analysis (synovial fluid SF)
MRI/CT
arthroscopy
Give an overview of synovial fluid
an ultrafiltrate of plasma and hyaluronic acid
Gross appearance:
Protein - increases with synovitis and increasing inflammation (>2.5g/dl abnormal, >4g/dl sepsis)
viscosity - finger test - 2.5-5cm stretch
ctyology - OA horse: 5000-10000 cells/ml, OA dos 500-5000cells/ml, <10%neutrophils
clot formation - rapidity and size roughly proportionate to severity of synovitis
mucinous precipitate quality - 0.5ml fluid into 2 ml 2% acetic acid, quality of clot and clarity of solution, not very accurate.
What are the main goals for treating OA
identify and treat underlying cause (abnormal forces on the joint, i.e. articular fracture, cruciate rupture, or normal forces on an abnormal joint i.e. osteochondrosis, HD)
block inflammatory cascade and permit repair if possible
pain relief and long term management
weight control and physical therapy
Give some examples and name the 2 groups of anti-inflammatories and analgesics
NSAIDs (Non Steroidal Anti-inflammatory drugs)
COX-2 inhibitors
side effects
potentially decrease PG synthesis
Dosing
Corticosteroids:
Intra-articular (equine)
Phospholipase inhibitors
Effects on cartilage metabolism
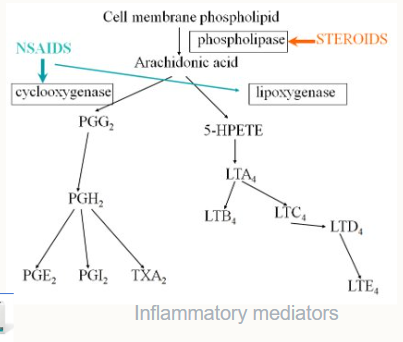
What do we need to be careful of with COX related drugs
Cyclo-oxygenase is needed in the brain, gut and kidneys. If we use COX-inhibitors we risk causing severe damage to these areas e.g. renal failure.
What are preventative measures we can take to reduce the risk of OA
maintain joint mobility and muscle strength
minimise additional joint destruction
weight management
what are some examples of adjunctive treatments
Hyaluronic acid - intra-articular, intra-venous, anti-inflammatory
Pentosan polysulfate (not a lot of evidence to support its use) - anabolic effect (build), HA synthesis, catabolic enzyme inhibitioin, clinical improvement
PSGAGs (also criticised, not a lot of evidence to support their use) - intra-articular, intra-muscular, oral, anti-inflammatory, HA synthesis, complications?
IRAP - interleukin-1 receptor antagonist protein, harvested from the animal’s own blood and put into the joint.
PRP - platelet rich plasma, autologous (produced by the animal’s own body), anti-inflammatory, harvested, spun, treated and put into the joint.
Stem cells (actually mesenchymal cells), put into the tendon and supposedly signal what’s wrong with the area and how to fix it.
ALWAYS QUESTION AND SCRUTINISE NEW DRUGS
What is Anti-nerve growth factor and what are some complications/questions around it at the moment
It’s a monoclonal antibody designed for dogs that neutralises the nerve growth factor (NGF), a key player in OA for dogs, so it reduces pain. It’s eliminated by the body with minimal involvement of the liver/kidney.
Bedinvetmab - administered monthly as an injection, saw a reduction in OA pain. Some mild reactions were seen (swelling and heat). well tolerated at the recommended dose, not additional side effects were observed at overdose.
What is an extreme way we can reduce the effect of OA
Surgery:
Correct the underlying tissue, HD, cruciate rupture, elbow dysplasia, OCD etc.
salvage procedure - femoral, head and neck ostectomy, joint replacement, arthrodesis
joint resurfacing (not proven)
Suggest some ideas for the future of OA care
synthetic matrix and/or mesenchymal stromal cells, subchondral bone
suppression of matrix degradation (curative?)
gene therapy - synthesis of natural inhibitors of cartilage damage
synthesis of cartilage matrix
Why does the body need a skeleton
structural support
protection of vital organs from trauma
locomotion
mineral reservoir
what are some limitations of bones
rigid
hard/brittle
unable to expand from within - limited growth potential, can only expand at existing surfaces
What makes up bone tissue?
matrix - inorganic and organic
cells - osteo- cytes/blasts/clasts
vascular spaces (areas that vessels go through the bone)
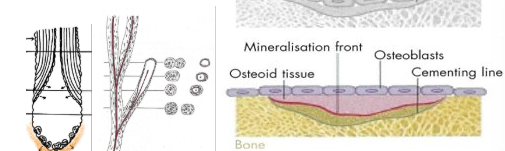
What is the organic component of the bone matrix - outline it.
OSTEOID = ground substance in which numerous collagen fibres are embedded - it’s what becomes bone (in the image it’s labelled). Over time it will harden and form proper bone
synthesised by osteoblasts
secreted onto existing bone surface
embedded in a ground substance of water and:
glycoproteins - osteonectin, osteocalcin (bind collagen and minerals)
proteoglycans - byglycan, decorin (bind growth factors)
bone sialoproteins - osteopontin, thrombospondin (associated with cell adhesion)
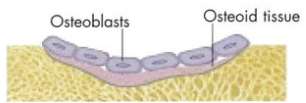
What is the inorganic bone matrix
bone minerals:
60-70% dry weight
confer hardness and rigidty
make bone radio-opaque
composed largely of crystals: hydroxyapatite, (Ca10(PO4)6(OH)2), carbonate, calcium phosphate.
How do the components of bone change over time?
younger - during birth, not proper bone yet (would seriously harm the mother if it was), also ensures that when the young fall, they don’t break lots of bones.
in humans at around 18-21, bones will be fully mature.
Older - more brittle
How does bone form?
Mineralisation
commences as soon as osteoid is secreted
reaches 70-80% final in around 3 weeks (in utero)
takes years to complete
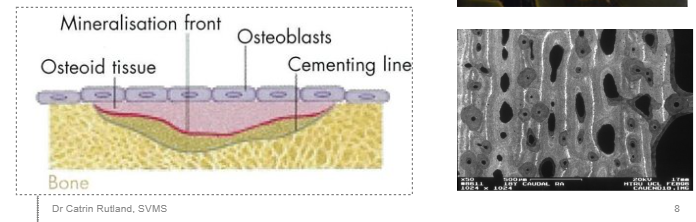
What are the two ways in which collagen fibres deposited, organise themselves?
woven bone - haphazard collage, ‘quick and dirty’ formation. Young growing animal, fracture, repair. Mineralises quickly. Looks woven, not a lot of strength.
Lamellar bone - parallel fibre bone. Thin layers of osteoid within which collagen fibres are parallel. Structurally superior. Collagen fibres deposited in different organisational structures.
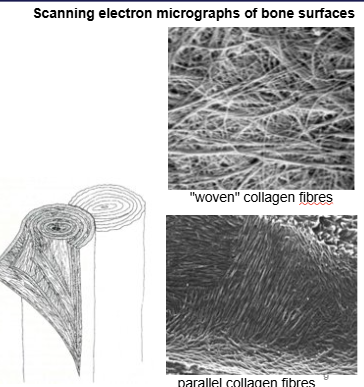
what does circumferential mean
slicing in half - used to look at bone microscopically.
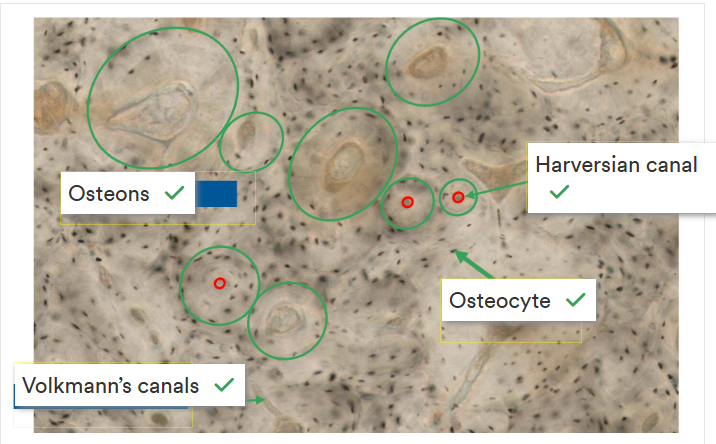
Outline osteons
A round structure in formation that has blood vessels in the middle
Forms tubes that run the length of the bone, pack together and provide strength to the bone.
Primary and secondary osteons
secondary osteons are formed off a primary one (e.g. motorway is the big main road, country roads come off it)
secondary osteons are more likely to move across the bone (width), transversely.
White arrows are the primary, black arrows are the secondary (hard to tell apart)
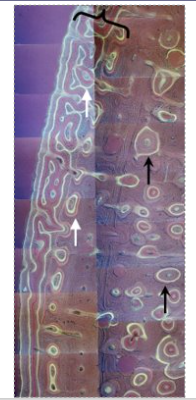
How does bone grow?
bone is living tissue, therefore it can be replaced and redistributed as loading requirements change.
grown from the inside, out.
outline primary osteons
formed in appositional bone growth, when bone increases in diameter.
Formed from existing blood vessels on the periosteum of the bone - the osteoblasts underneath the blood vessels in the periosteum have a decreased rate of osteoid production, whereas other side has an increased rate
Once ridges form, they fuse and form a tunnel around the blood vessel lined with the endosteum
Concentric lamellae formation: osteoblasts in endosteum build them onto the valls of the tunnel, filling inward until just enough space for the blood vessel
circumferential lamellae form as the bone continues to grow outward as the osteoblasts in the periosteum build them
Run parallel to the acix of the bone, contain one or more vascular canals are are ALWAYS surrounded by woven bone.
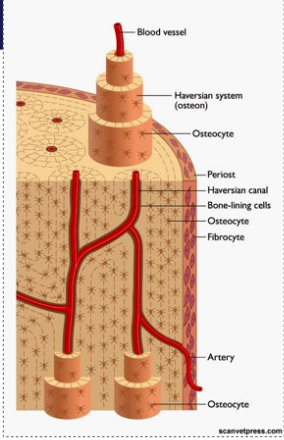
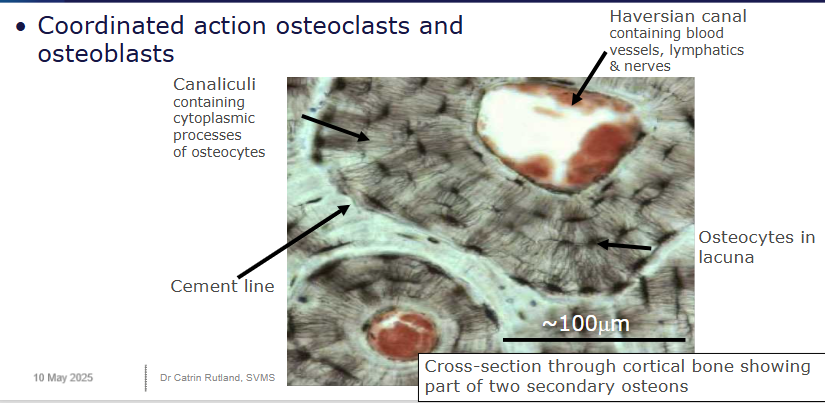
Outline secondary osteon structure
Contain a haversian canal (contains the nerves, blood and lymphatic vessels), canaliculi (contain cytoplasmic processes of osteocytes), cement line (around the whole osteon) and osteocytes (within lacuna(e)).
formed from an primary osteon
often formed due to remodelling needs
both primary and secondary have similar structure
What can we also call a Haversian system
osteon
and vice versa
Outline bone cells
Osteoblasts
derived from mesenchymal stem cells
synthesise and secrete osteoid
active in mineralisation process
Osteocytes
scattered within the matrix
interconnected by dendritic processes
derived from osteoblasts but stopped synthesis of matrix and dividing
reside within ‘lacunae’ which are interconnected by ‘canaliculi
long-lived
maintain the matrix
Osteoclasts
responsible for bone resorption
large, multinuclei cells
release protons, leads to an acid environment, leads to demineralisation
secrete proteases that destroy the organic matrix
derived from bone marrow
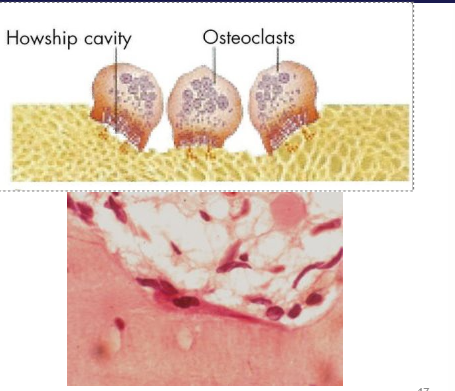
How is bone modelled/remodelled
Cellular mechanisms
coordinated action of osteoblasts/clasts
bone is excavated - osteoclasts excavate a cylindrical tunnel from a cutting cone (50 micro metres a day) - parallel to other osteons
bone is replaced - osteoblasts follow, form concentric lamellae of lamellar bone on walls, surrounding a centrally in-growing blood vessel
form a secondary osteon
facilitates: change in shape, material, repair of damaged bone, release of mineral ions
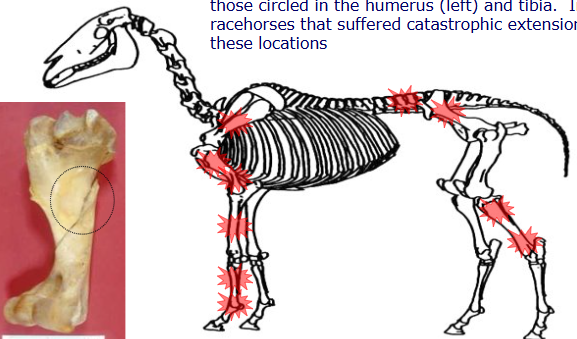
What are and what causes stress fractures
a syndrome involving localised bone injury associated with fatigue damage subsequent to repetitive loading/mechanical stress
common in performance animals
underlying cause of lameness
Over time:
may lead to a catastrophic break (shatter/break completely) over time.
How can we locate stress fractures?
Micro CT - very high resolution imagine (can’t be used on anything high alive).
Often can’t be seen on X-ray - will see behavioural signs of discomfort.
How does the body try to repair stress fractures?
Osteoclast will burrow in the area of the fracture, creating more holes so that nutrients can reach the area for it to start healing
this in turn makes the bone even weaker before it can start healing
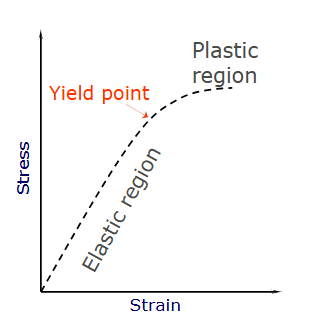
outline the pathophysiology of stress fractures using this image
stress = force per unit area
strain = percentage of elongation
stiffness = resistance to deformation
elastic region = measure of elasticity
yield point = point at which the structure will no longer return to its original shape
plastic region = structure deformed and moving towards failure.
fatigue = progressive decline in mineral properties over time
cyclical loading of quite intense loads may steadily weaken the bone
we can predict with race horses, how many steps they need to take before obtaining a stress fracture - look at strain vs number of cycles to failure
the higher intensity of training, the less time it will take to reach that dangerous point.
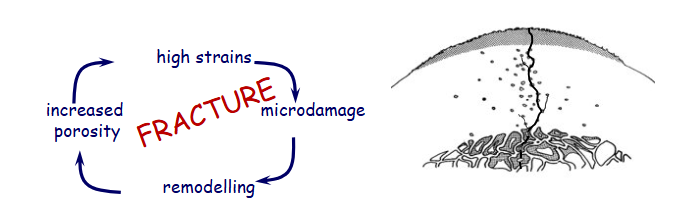
why is microdamage dangerous
causes structural damage at various levels
can cause cell death
can cause vascular disruption
leads to remodelling of the bone, which if continuously re broken, will lead to the catastrophic break.
Where are common locations for stress fractures?
How can we diagnose/manage stress fractures
History:
intense training?
none - ‘incidental finding’
none - spontaneous catastrophic fracture
subtle loss of performance
acute-onset lameness associated with work
Modify exercise patterns:
combination of load, repetition and inadequate recovery will lead to a stress fracture
essential feature of treatment is to break the cycle (change intensity, level and/or type of exercise)
Where do we find cartilage
Joints - flexible interface b/w bones, smooth bearing surface
flexible support - outer ear, sternum, larynx, tracheal rings
How does cartilage grow?
Interstitially
difficult as cartilage is avascular and aneural
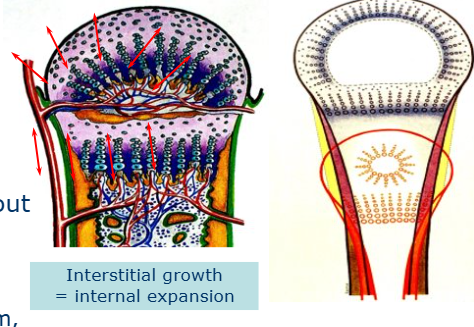
What does matrix include
NOT just osteoid without the mineral
different biochemistry, microstructure, metabolism and cell types found
avascular and aneural
Outline hyaline/articular cartilage
Typical form of cartilage
Found:
joint surfaces including synovial joints and sternum
precursors to bone in embryonic skeleton (embryonic ossification)
present inside bones, serving as a centre of ossification or bone growth
predominantly type 2 collagen.
Function:
withstand and distribute load
act as an elastic shock absorber
provides a wear resistant surface to articulating joints
self maintaining
avascular, aneural and alymphatic
what does Alizarin red stain do?
stains cartilage
used to see cartilage as a precursor in foetuses.

Outline fibrocartilage
white fibrocartilage
found in areas requiring tough support/great tensile strength e.g. intervertebral disks, symphyses
lines surface of bony grooves for tendons - flexor digital sheath, palmar surface of navicular bone
interface ligament/tendon and bone
contains more collagen than hyaline cartilage
contains type 1 and 2
lacks a perichondrium
outline elastic cartilage
yellow/elastic cartilage
found in pinna and several tubes (auditory and eustachian canals, larynx, epiglottis and arytenoids)
keeps tubes permanently open
similar to hyaline but contains elastic bundles (elastin) scattered throughout the matrix
what are chondrocytes
the only cells found in cartilage
produce and maintain the cartilage matrix
low density
obtain nutrition and O2 by diffusion
chondrocytes continue secreting new matrix when embedded in the matrix (interstitial growth, internal expansion)
chondrocytes are capable of division within the matrix
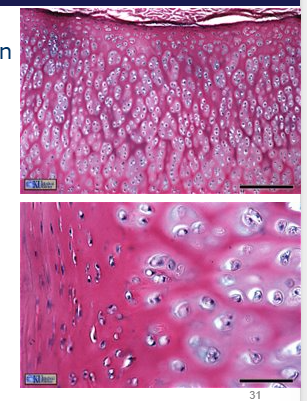
How many kinds of chondrocytes are there
4 - superficial, middle, deep and calcified
all have distinct biochemical and physiological properties.
morphology varies with depth - see image
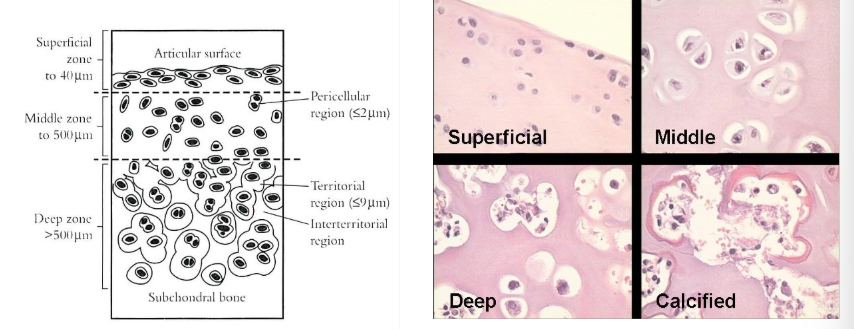
What about hyaline articular cartilage structure?
characteristic zonal microstructure
collagen framework embedded in a highly hydrated ground substance (carbohydrate and water rich)
type 2 collagen - 50% dry weight (type 1 collagen in fibrocartilage ± elastic fibres in elastic cartilage
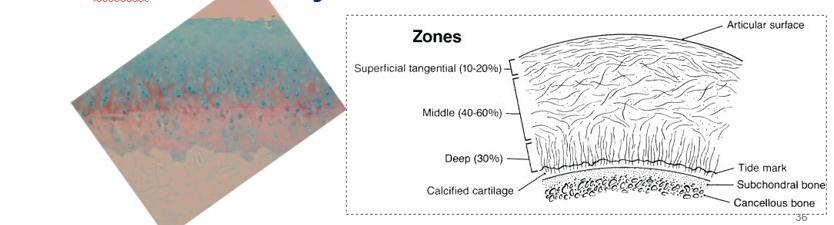
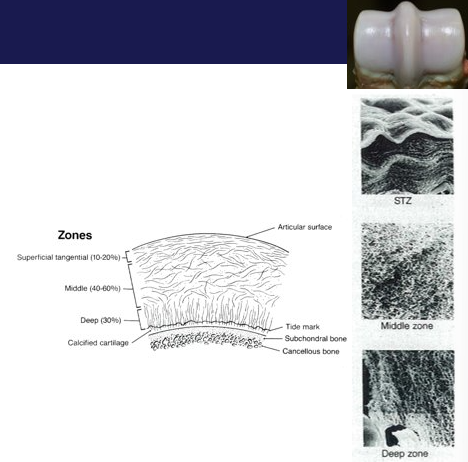
What is the 2-phase composite of articular cartilage
3D latticework of type 2 collagen fibres, orientation of fibres is divided into zones, deeper (interface with bone) layer calcifies
Hydrated gel (chondrocytes, no blood vessels, lymphatics or nerves, deeper portion calcifies)
fills (expands) space b/w collagen fibres
held together by other ‘structural’ molecules such as collagen XI, collagen IX, fibromodulin, decorin, COMP.
What is aggrecan
a proteoglycan
core protein
glycoaminoglycan chains form
around 10,000 negative groups
attracts Ca+
osmotic (pulls water in)
linked to long hyaluronan to form long chains
How do our joints resist compression?
articular cartilage - VISCOELASTIC
allows the ground substance to move in and out of the gaps
moves out when pressure is applied
moves back in when pressure is released
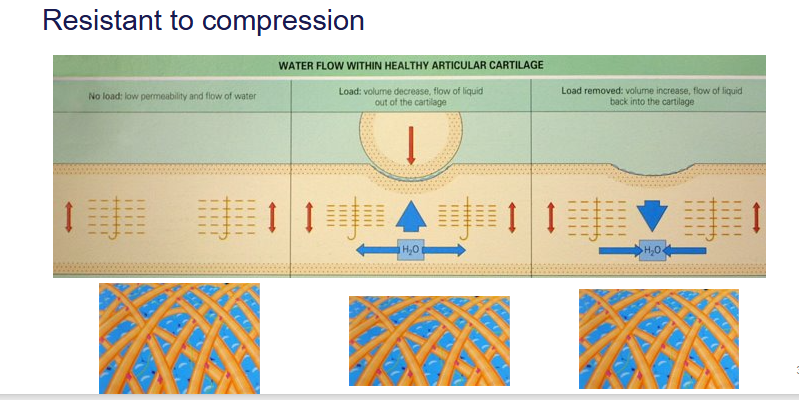
What can a high strain rate (as we see for race horses) lead to?
The cartilage snaps
doesn’t have enough time to move to allow the water molecules out
the molecules force themselves out due to the pressure
results in the cartilage breaking
What is a cement line
junction betwen outermost lamellae of new osteon and some pre-existing older bone
contains a lot of proteoglycan that stick the old and new bone together
What other kinds of fractures are there?
hairline
greenstick
transverse
spiral
comminuted
compound
outline how stress fractures lead to catastrophic injuries (general cycle)
fatigue damage leads to microdamage
cyclical loading leads to an accumulation of microdamage
leads to a stress fracture
What happens to the forces on the limb at rest
horses rest standing most of the time
equal balance of body weight
forelimbs carry 58.6% and hind limbs carry 41.4%
bones will be slightly compressed
what happens to the forces on the limb during locomotion
force distribution varies in accordance with the sequence and timing with limb contacts and overlaps
how they change their gate depending on activitiy carried out
different forces acting on the bones (gravitational etc.)
How do bones deform when at rest
strength of the bone can be weakened and cause deformation when not exercising
overweight, meaning the load on the joints is too heavy resulting in damage
generally, less deformation than during locomotion
elastic deformation at rest
how do we get bone deformation during locomotion
bones can change shape and remodel during locomotion which can lead to deformation to try and strengthen, need time to recover
various types on different joints around the body
more likely to cause plastic deformation than elastic at rest
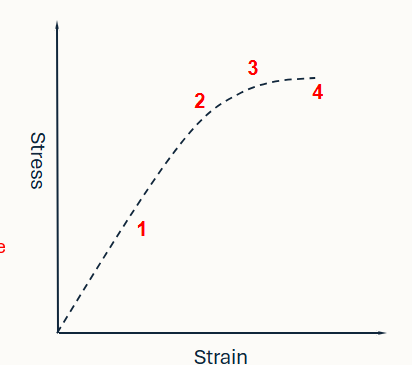
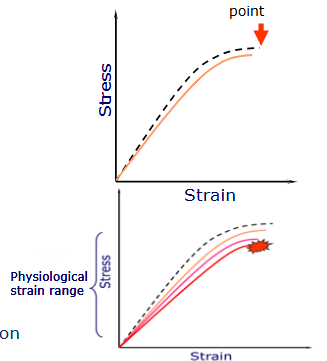
Identify the regions on this graph
Elastic region - measure of elasticity (return to original shape)
yield point - point at which the structure will no longer return to its original shape
plastic region - structure is deformed and moving towards failure
failure point- where the material breaks
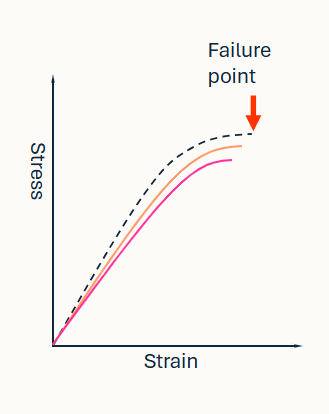
What is happening in this graph
Fatigue:
a progressive decline in material properties (pink and orange line)
failure point occurs at lower stress with repetition, as the bone weakens
cyclical loading of quite intense loads may steadily weaken the bone