Chapter 5: Biological Molecules
1/58
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
59 Terms
the role of dehydration reactions in the formation and breakdown of polymers.
If a water molecule is lost, it is known as a dehydration reaction (synthesizing a polymer). For example, carbohydrate and protein polymers are synthesized by dehydration reactions.
the role of hydrolysis reactions in the formation and breakdown of polymers.
Hydrolysis means water breakage (breaking down a polymer). The bond between monomers is broken by the addition of a water molecule, with a hydrogen from water attaching to one monomer and the hydroxyl group attaching to the other.
visualization of a dehydration synthesis reaction (is water leaving and a bond forming?)
Dehydration removes a water molecule, forming a new bond.
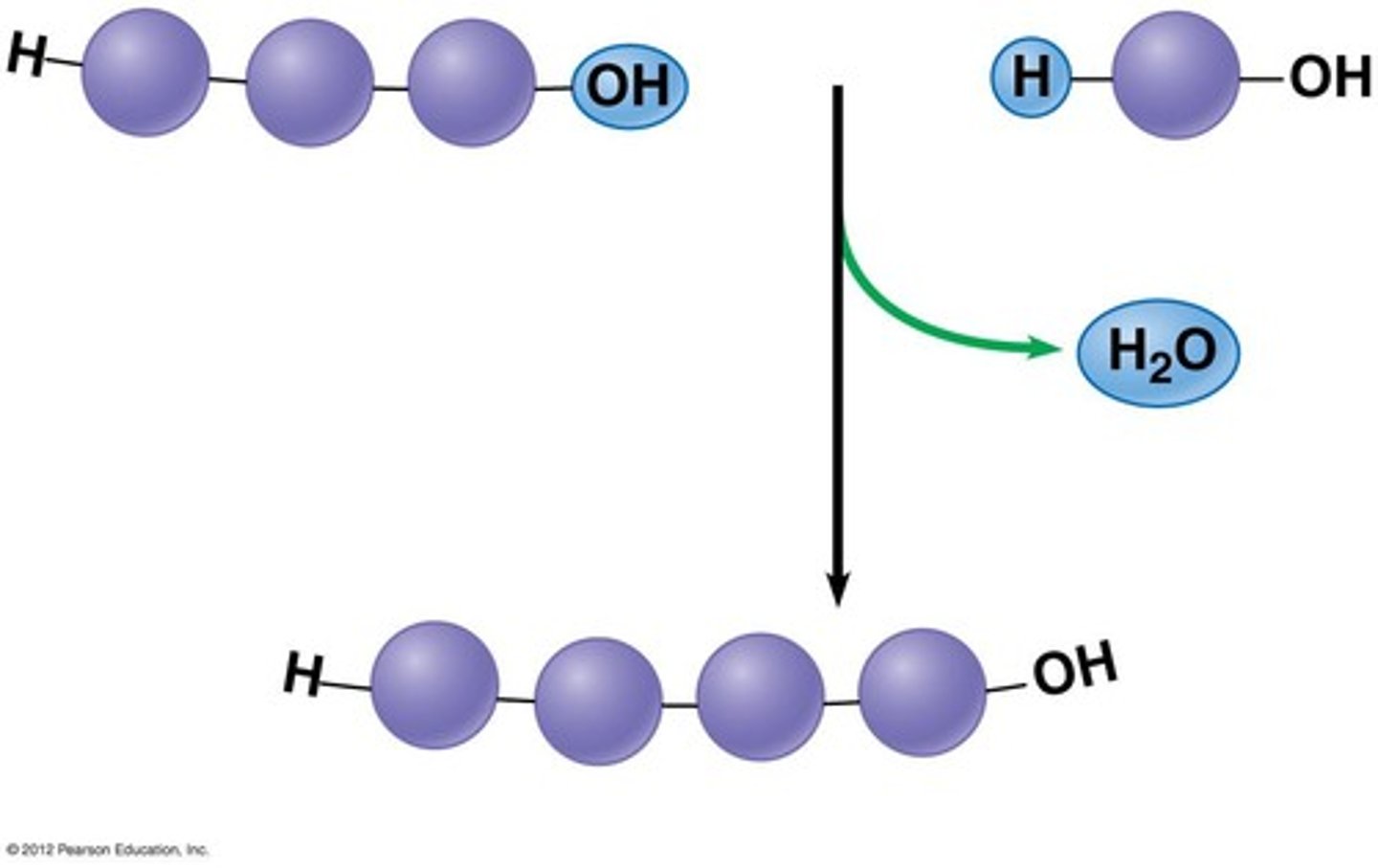
visualization hydrolysis reaction (is water coming in and a bond breaking?)
Hydrolysis adds a water molecule, breaking a bond
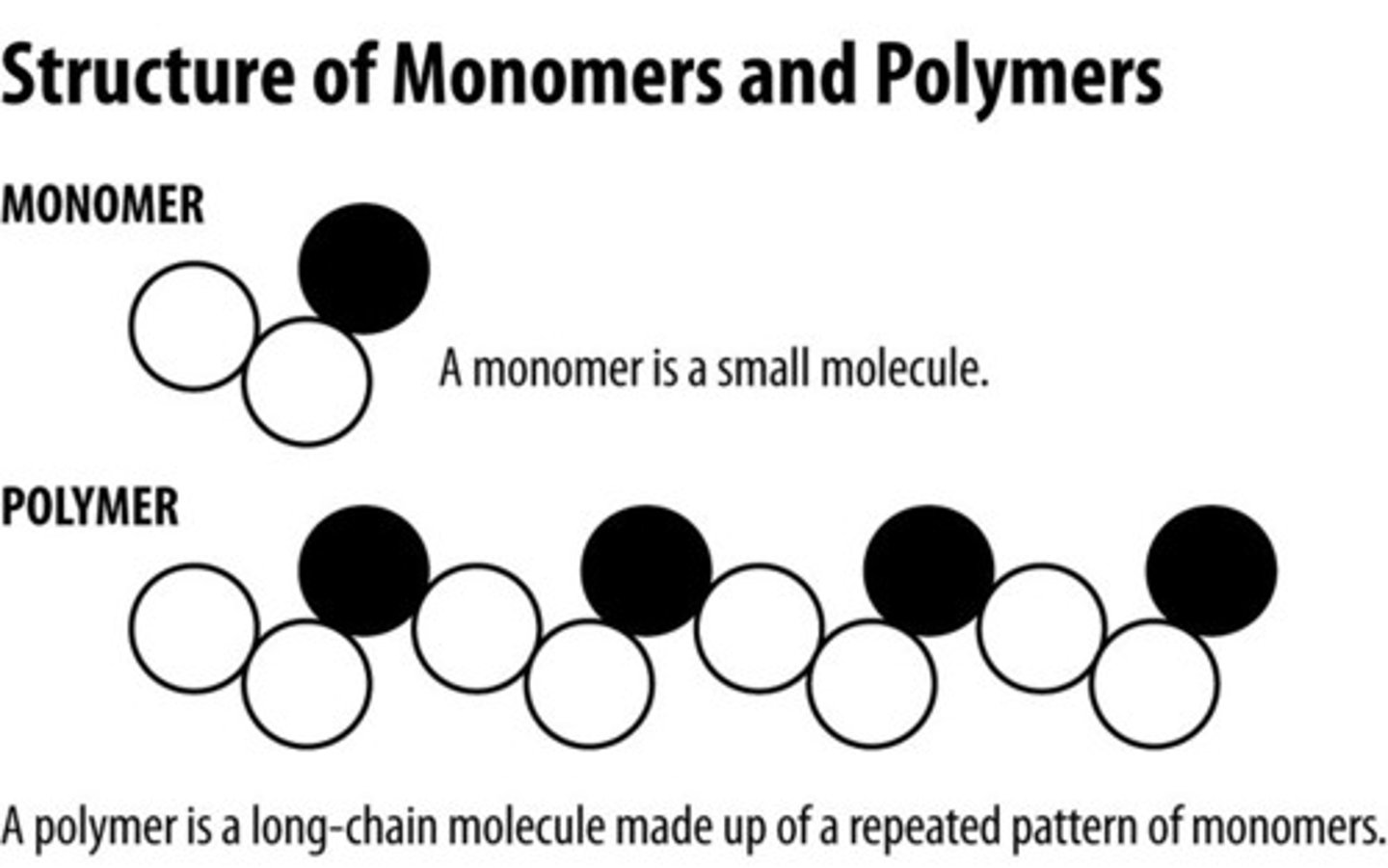
monomer
The repeating units that serve as the building blocks of a polymer and are smaller molecules
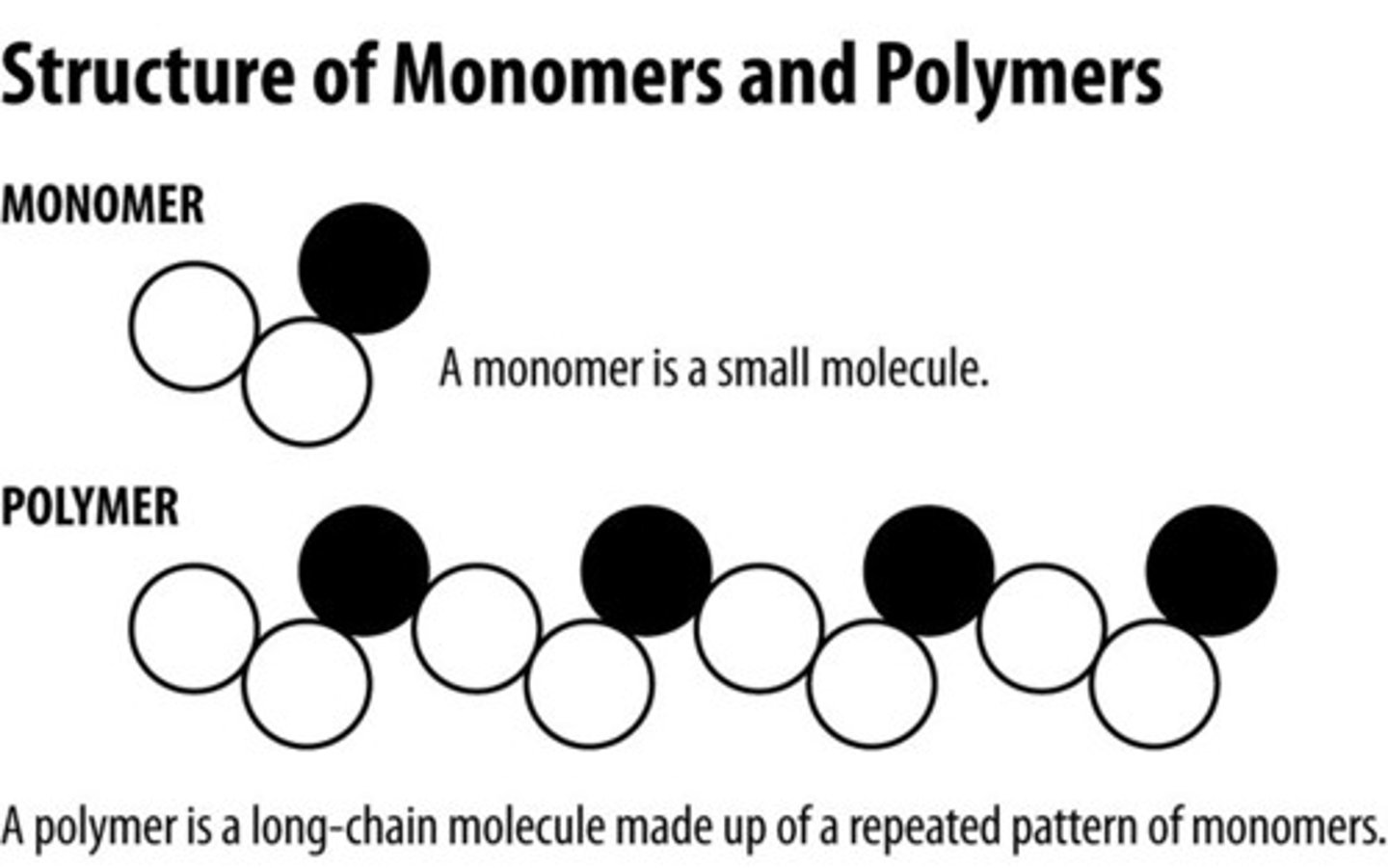
polymer
a long molecule consisting of many similar or identical building blocks linked by covalent bonds, much as a train consists of a chain of boxcars.
bond involved in connecting the monomers
The reaction that connects a monomer to another monomer or a polymer is a condensation reaction, a reaction in which two molecules are covalently bonded to each other with the loss of a small molecule
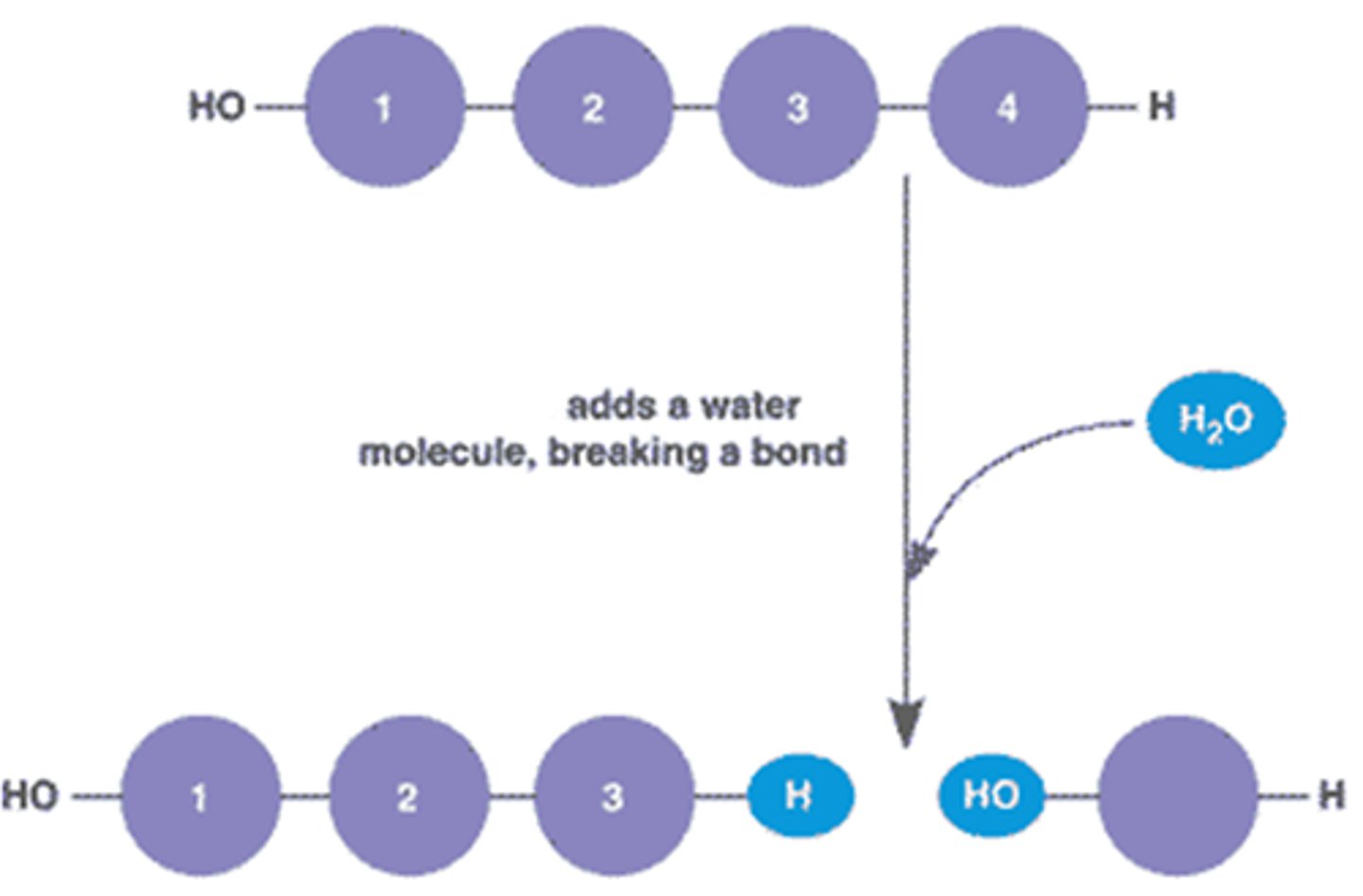
What process results in the destruction of a polymer?
The process that results in the destruction of a polymer is called hydrolysis. This process involves the addition of a water molecule to a polymer, which breaks the bond between monomers, resulting in two shorter polymers.
Which out of the four macromolecules does not involve monomers and polymers?
Lipids are the one class of large biological molecules that does not include true polymers, and they are generally not big enough to be considered macromolecules

carbohydrates
Serve as a primary energy source and provide structural support.
Lipids
Provide energy, form cell membranes, and act as hormones.
Proteins
Perform a wide range of functions, including catalyzing reactions and transporting substances.
Nucleic Acids
Store genetic information and are involved in gene expression.
an example of a function (role) for each type of large biological molecule
Carbohydrates:Function: Serve as a source of energy and provide structural support.
Proteins:Function: Perform a wide range of functions, including catalyzing reactions and transporting substances.
Nucleic Acids:Function: Store and transmit genetic information.
Lipids:Function: Provide energy, form cell membranes, and act as hormones.
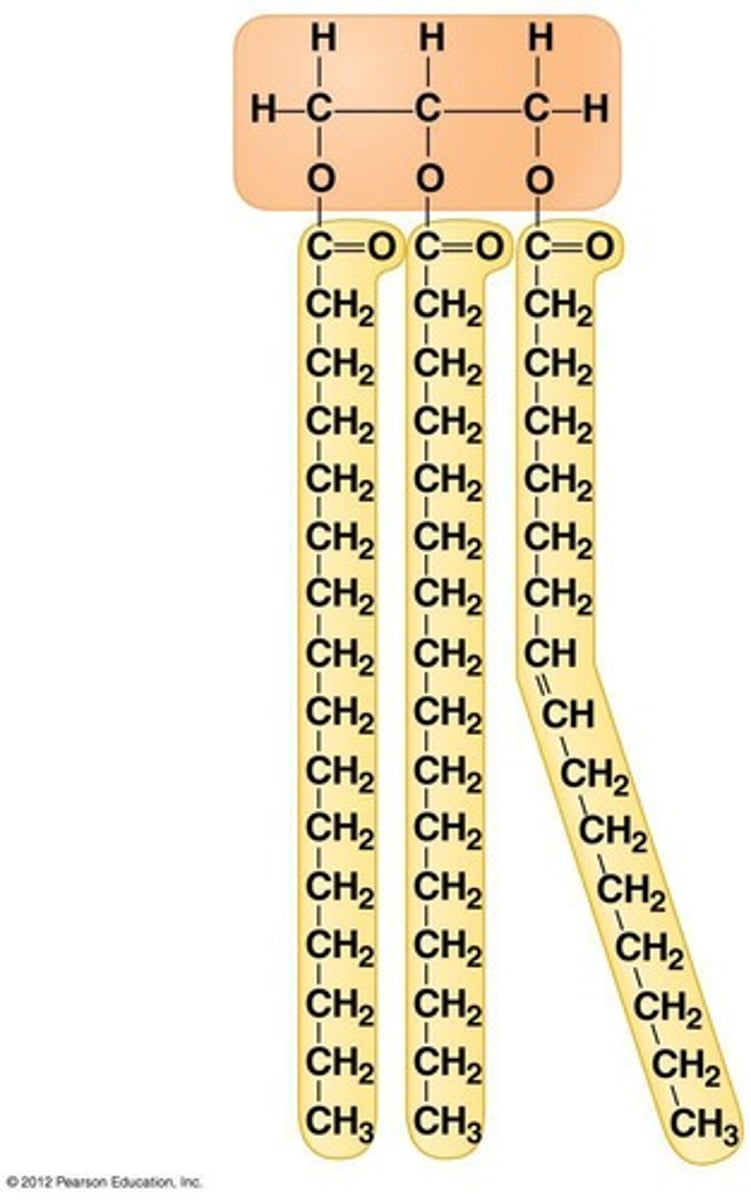
triglycerides
A fat molecule consisting of three fatty acids linked to glycerol
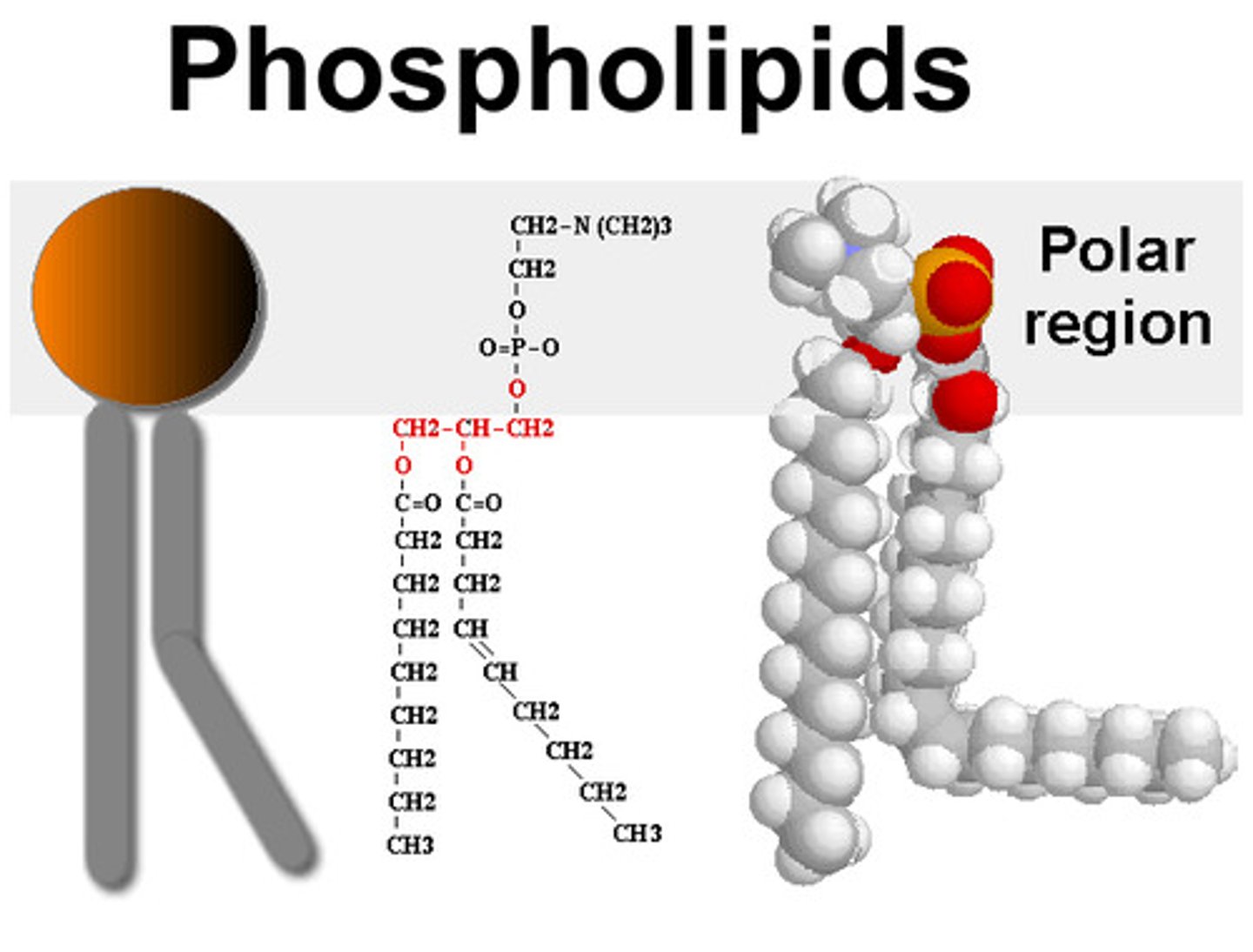
phospholipids
Phospholipids are essential lipids that form the foundation of cell membranes
steroids
steroids are a class of lipids distinguished by a carbon skeleton with four fused rings

Which of the three would you call a "storage lipid"
triglycerides
Which are used to form membranes
phospholipid
Which are signal molecules?
Signal molecules are crucial for communication between animal cells. They are classified based on the type of secreting cell and the route taken to reach their target. Phospholipids act as a signal molecule.
the basic chemical makeup of triglycerides. How does this relate to hydrophobicity?
The hydrophobic behavior of triglycerides is similar to that of other lipids, which also have non-polar carbon-hydrogen bonds that do not interact with water. This property is crucial for the formation of lipid bilayers in cell membranes, providing a barrier that separates the cell's interior from its external environment.
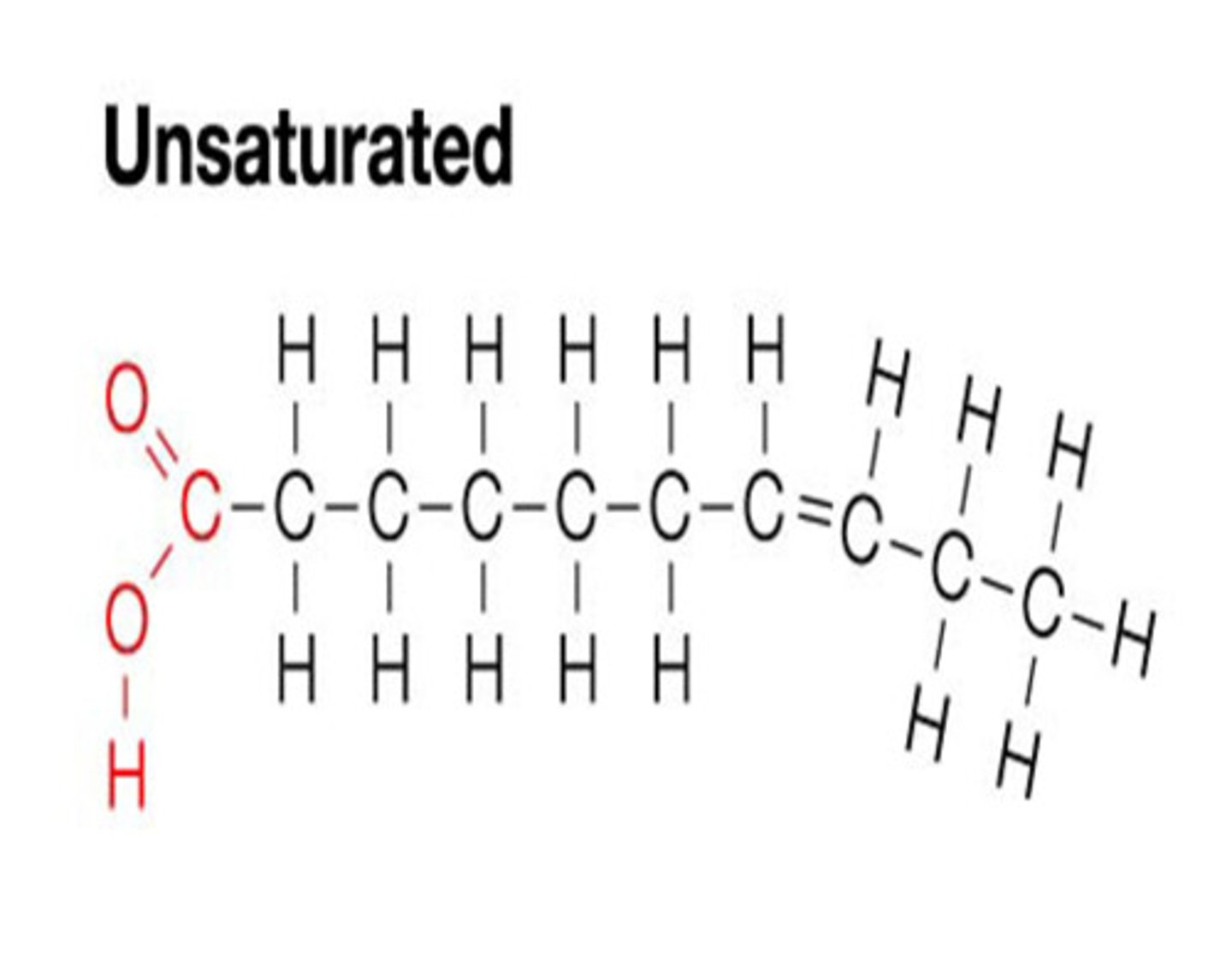
Unsaturated Fats:
contain one or more double bonds in their hydrocarbon chains. Each double bond reduces the number of hydrogen atoms that can attach to the carbon skeleton. These fats are usually liquid at room temperature, like olive oil, because the double bonds create kinks in the chains, preventing tight packing.
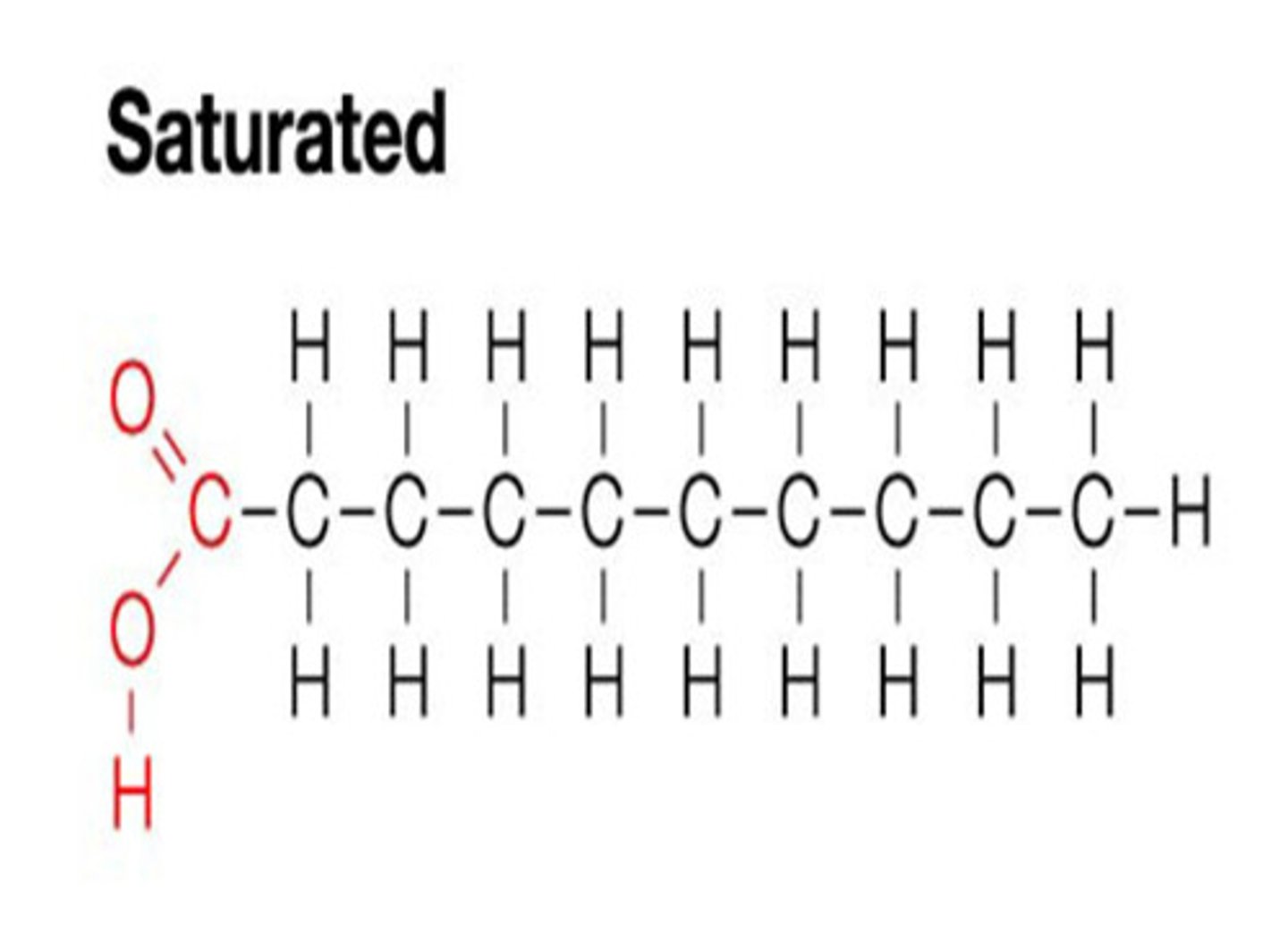
saturated fats
Saturated fats have no double bonds between carbon atoms in their hydrocarbon chains.
At room temperature, saturated fats are typically solid, like butter, because their straight chains allow them to pack closely together. This allows each carbon atom to bond with the maximum number of hydrogen atoms, making the chain "saturated" with hydrogen.
Which has one or more double bonds
unsaturated bonds
How does this affect structure
create a kink or bend in the fatty acid molecule, primarily due to the cis configuration of naturally occurring double bonds
Which tends to be liquid at room temperature? Solid? Why?
Saturated fats have hydrocarbon chains without double bonds, allowing them to pack tightly. This close packing makes them solid at room temperature, like butter and lard. Unsaturated Fats have one or more cis double bonds, creating kinks in their chains. The kinks prevent tight packing, making them liquid at room temperature, like olive oil.

How does this relate to the health impacts of unsaturated vs. saturated fats?
Unsaturated fats are generally healthier than saturated fats because they can help improve cholesterol levels, reduce heart disease risk, and support overall well-being, while saturated fats tend to raise bad cholesterol (LDL) and are linked to an increased risk of heart disease
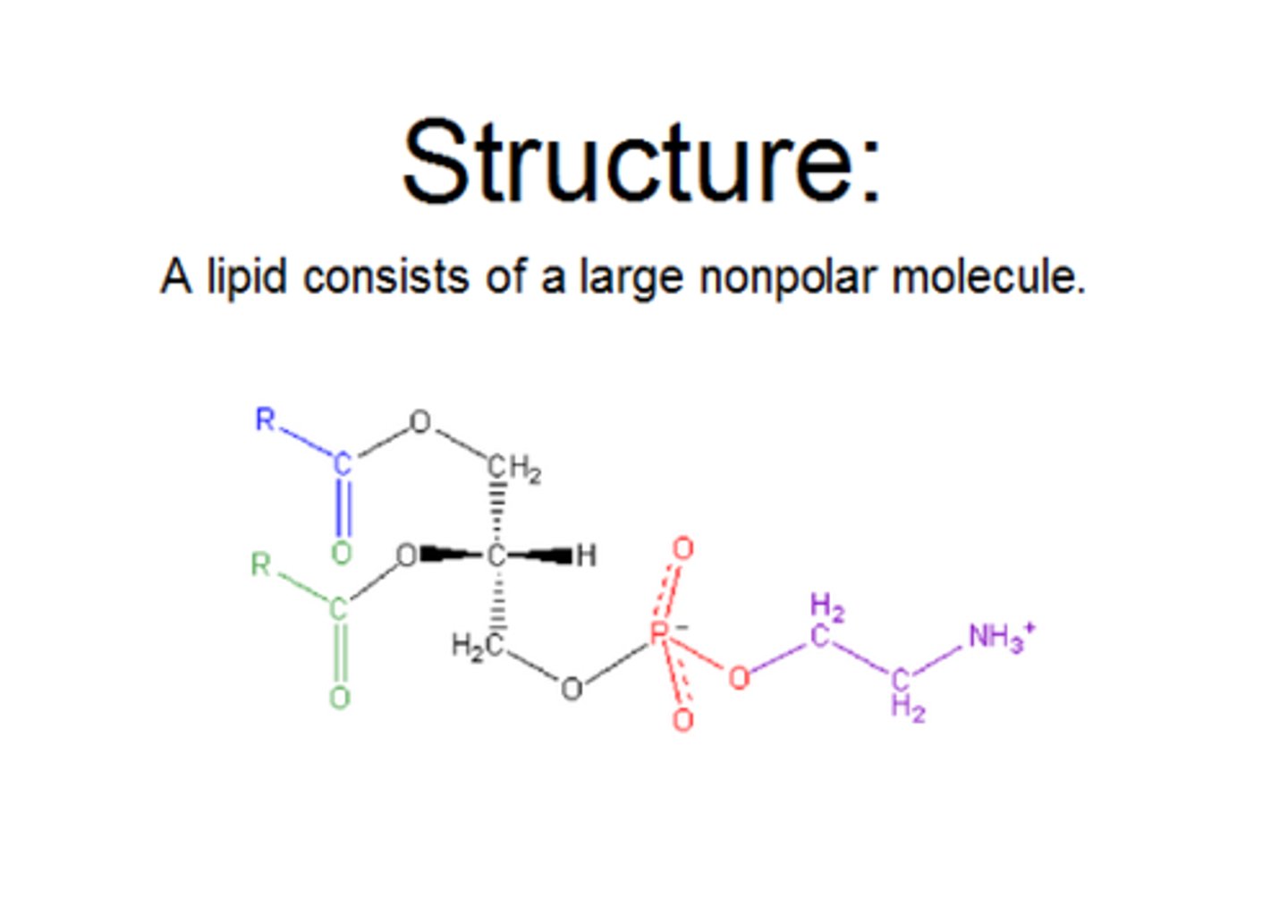
the basic structure of a phospholipid.
A phospholipid is an amphipathic molecule, meaning it has both a hydrophilic ("water-loving") head and two hydrophobic ("water-fearing") tails. The head consists of a phosphate group linked to glycerol, often with an additional small charged or polar molecule like choline. The tails are composed of fatty acids, one of which typically has a kink due to a cis double bond.
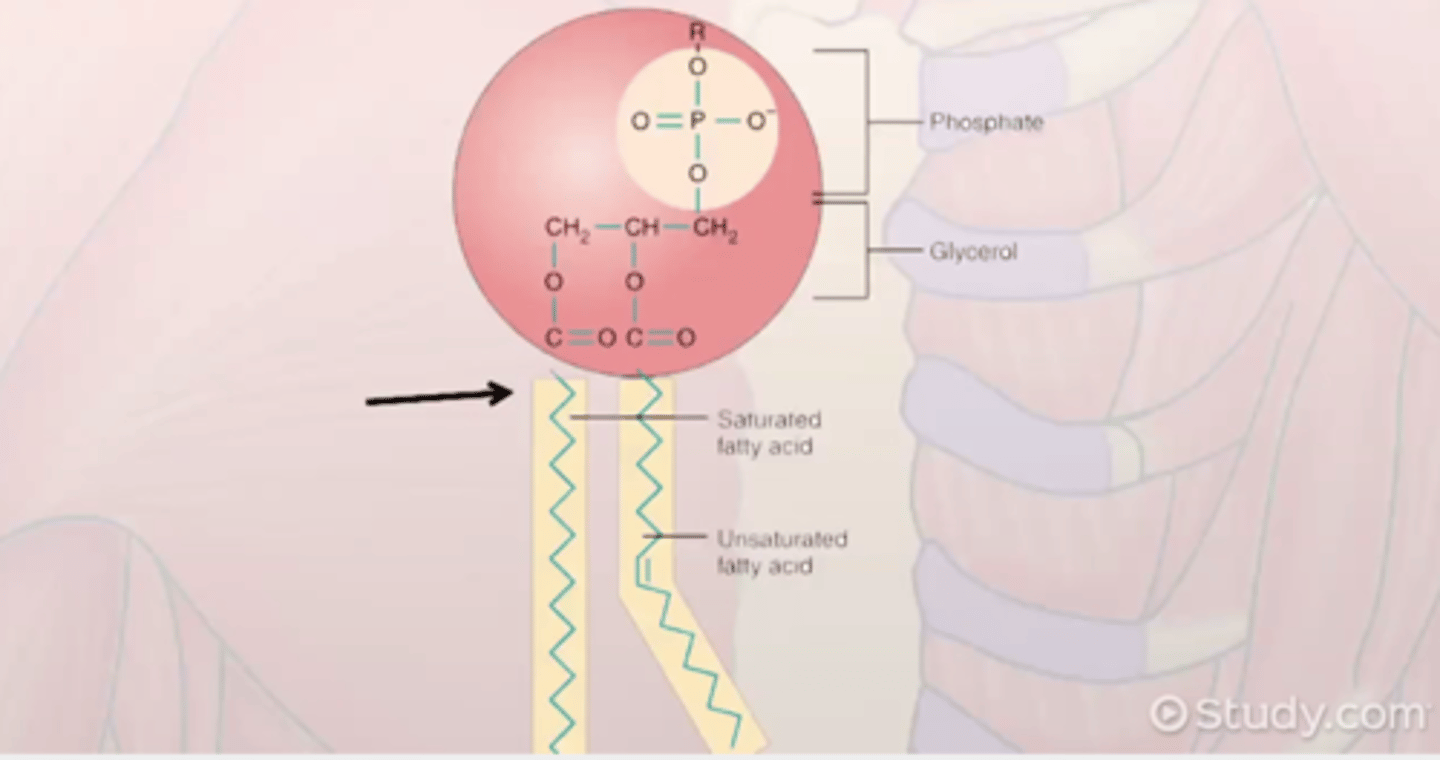
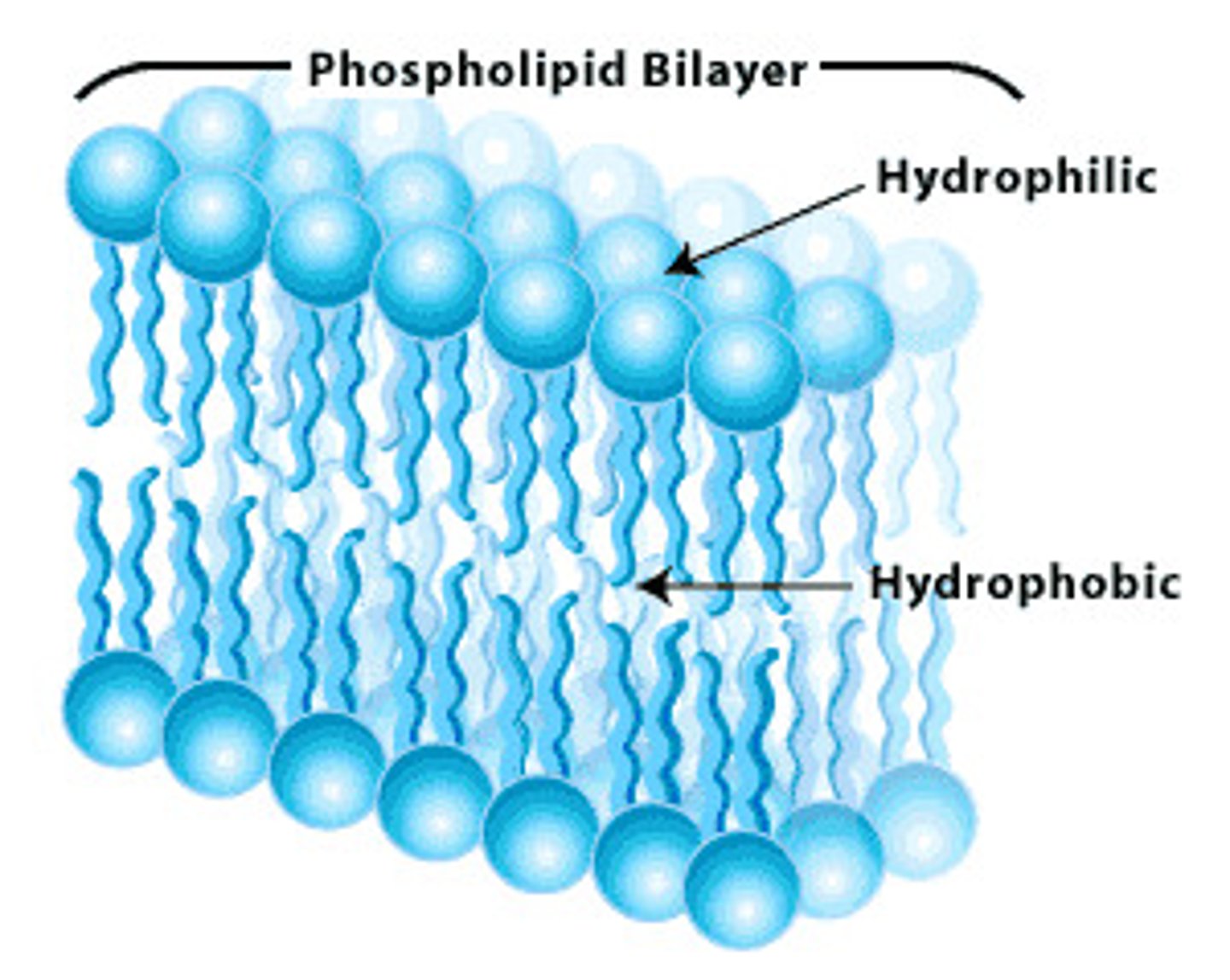
How does the basic structure of a phospholipid relate to the phospholipid bilayer that makes e.g. cell plasma membranes?
A phospholipid's basic structure, with its water-loving (hydrophilic) phosphate head and water-fearing (hydrophobic) fatty acid tails, is directly responsible for the formation of the phospholipid bilayer in cell membranes
the basic structure of a steroid.
Steroids are lipids characterized by a carbon skeleton consisting of four fused rings. This structure includes three six-sided rings and one five-sided ring, forming a unique backbone that is common to all steroids.
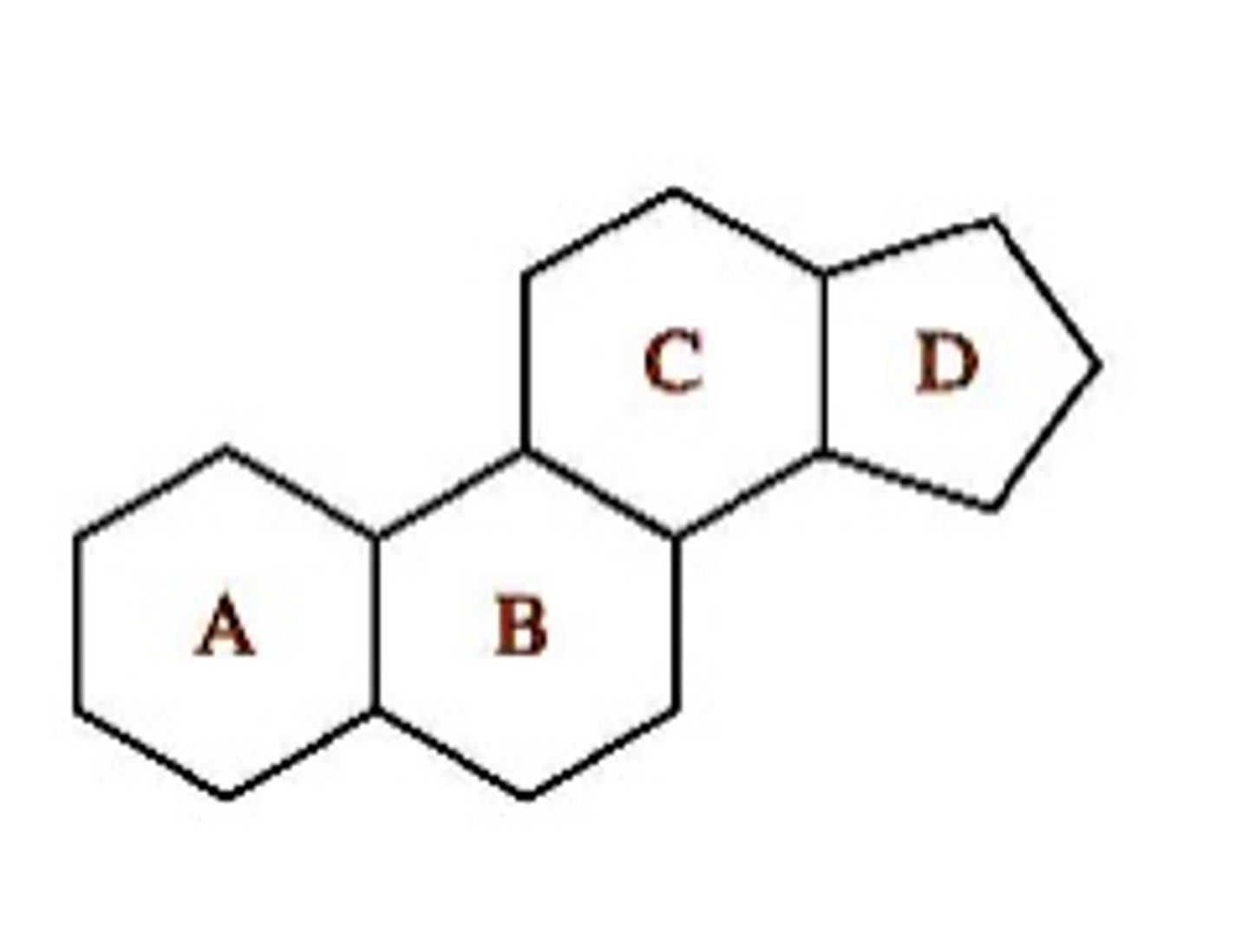
How does the structure of steroids relate to their function as signaling molecules?
Steroids function as signaling molecules due to their lipid-soluble nature, allowing them to pass through cell membranes easily. Once inside the cell, they bind to intracellular receptors, forming a hormone-receptor complex.
the structures of monosaccharides
Monosaccharides are the simplest form of carbohydrates, often referred to as simple sugars. They generally have molecular formulas that are multiples of the basic unit .
the structures of disaccharides
Disaccharides are carbohydrates composed of two monosaccharides linked by a glycosidic bond, formed through a dehydration reaction
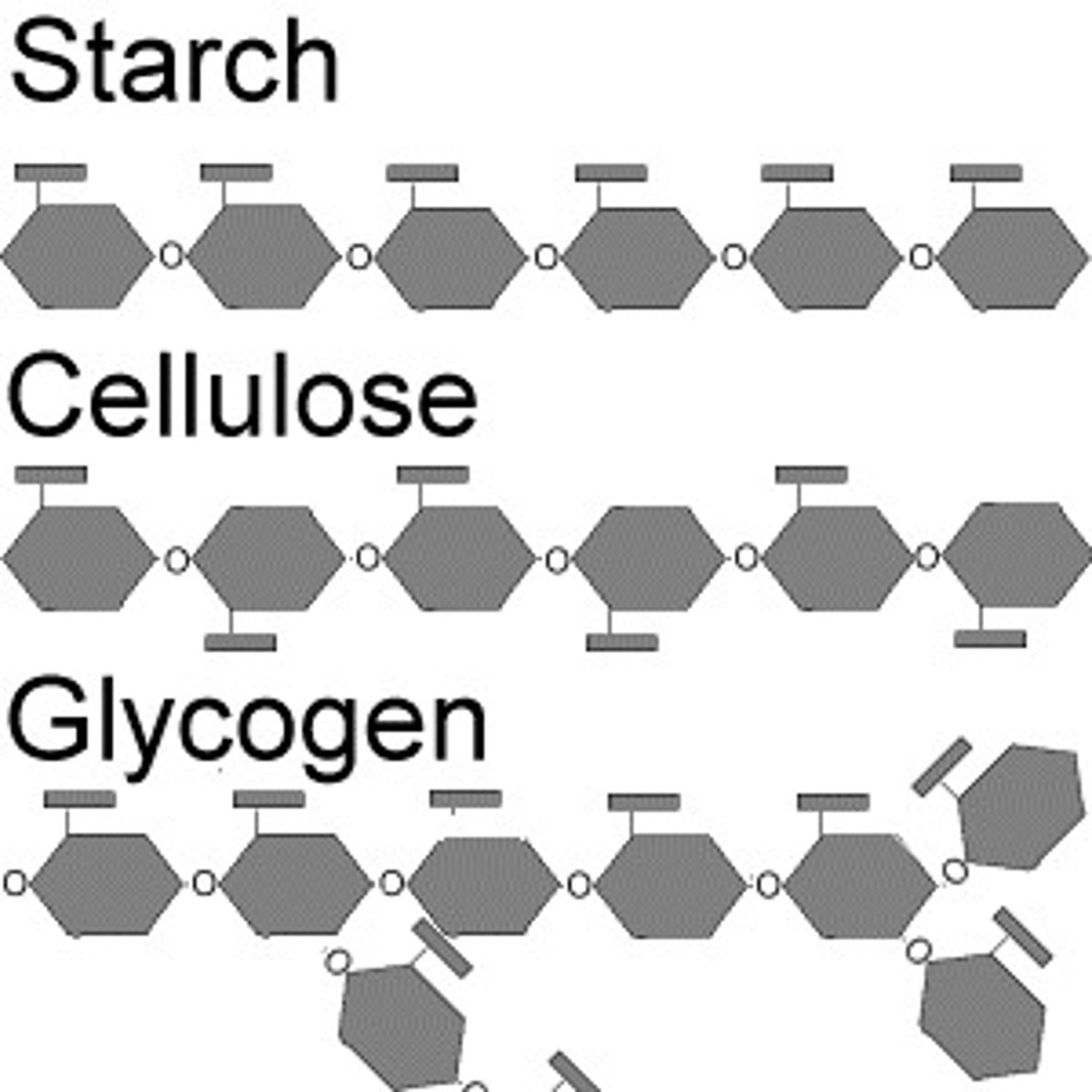
the structures of polysaccharides
Polysaccharides are large macromolecules composed of hundreds to thousands of monosaccharides linked by glycosidic bonds. Their structure and function are determined by the types of monosaccharides involved and the positions of these linkages.
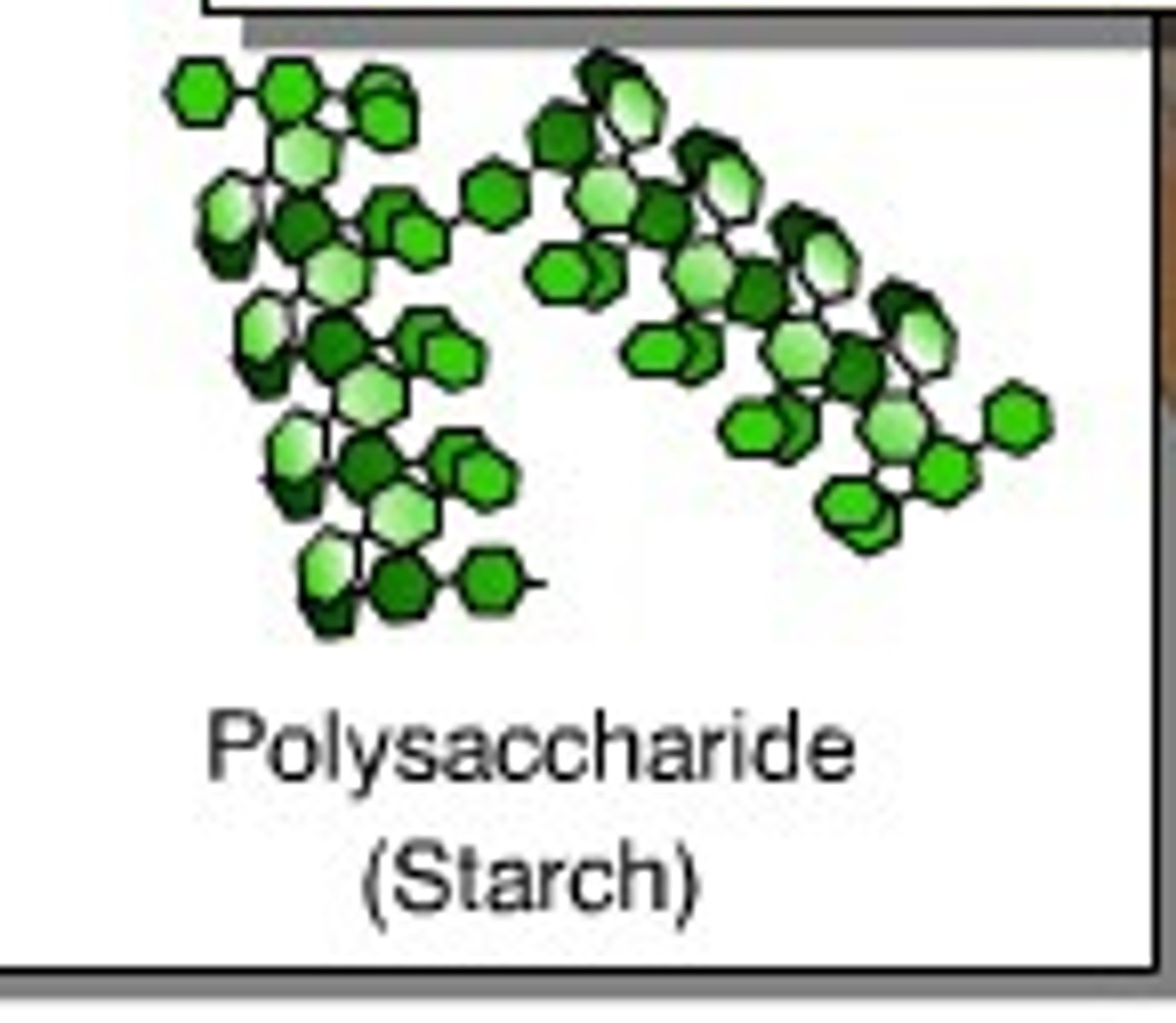
Which of these is a monomer? polymer?
Monosaccharides are monomers and Polysaccharides are polymers.
What are some of the functions of sugars in the human body?
energy source, storage, structural role, metabolic intermediates
What are some different functions of carbohydrates?
fuel and energy storage, structural support, and cell-cell recognition
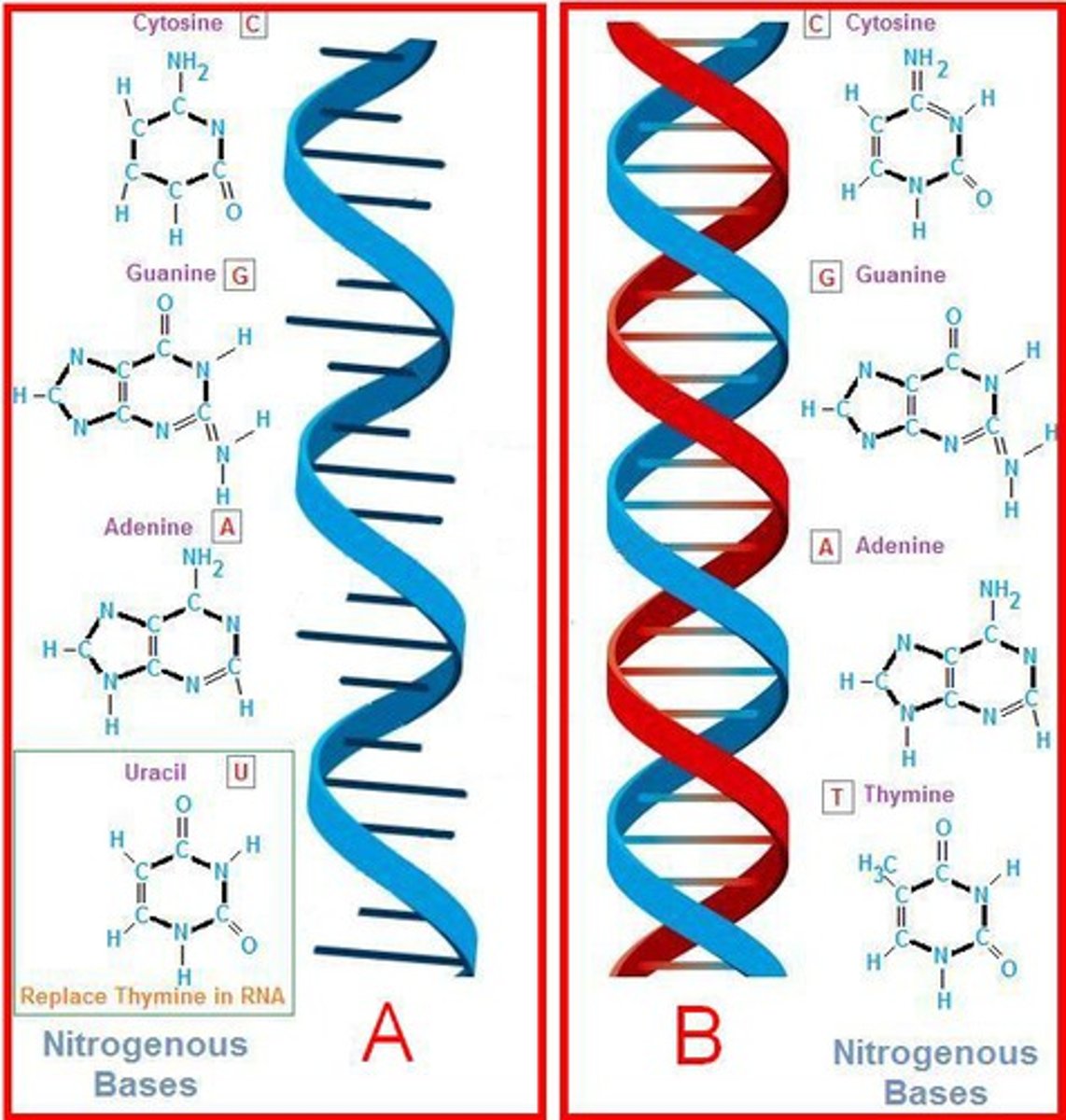
the structures of nucleotides and nucleic acid polymers
Nucleic acids, such as DNA and RN.Each nucleotide has three key components: Nitrogenous Base, five carbon sugar (pentose), and phosphate group
What parts of the nucleotides make up the "backbone"?
the "backbone" is formed by a repeating pattern of sugar and phosphate groups. This structure is known as the sugar-phosphate backbone
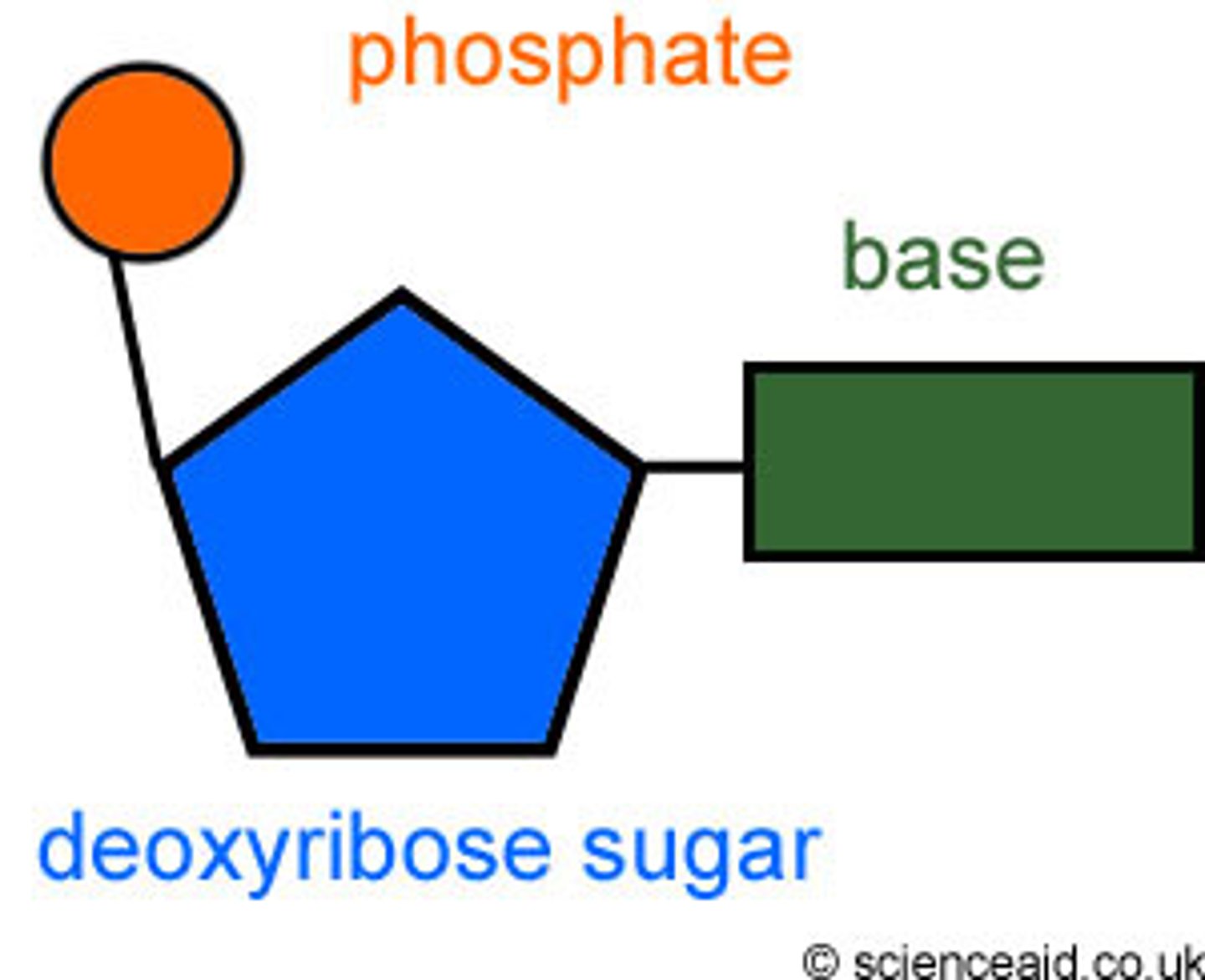
What type of bond connects nucleotides?
Nucleotides are connected in a polynucleotide chain by phosphodiester linkages. This type of bond involves a phosphate group that covalently links the sugar molecules of two adjacent nucleotides.
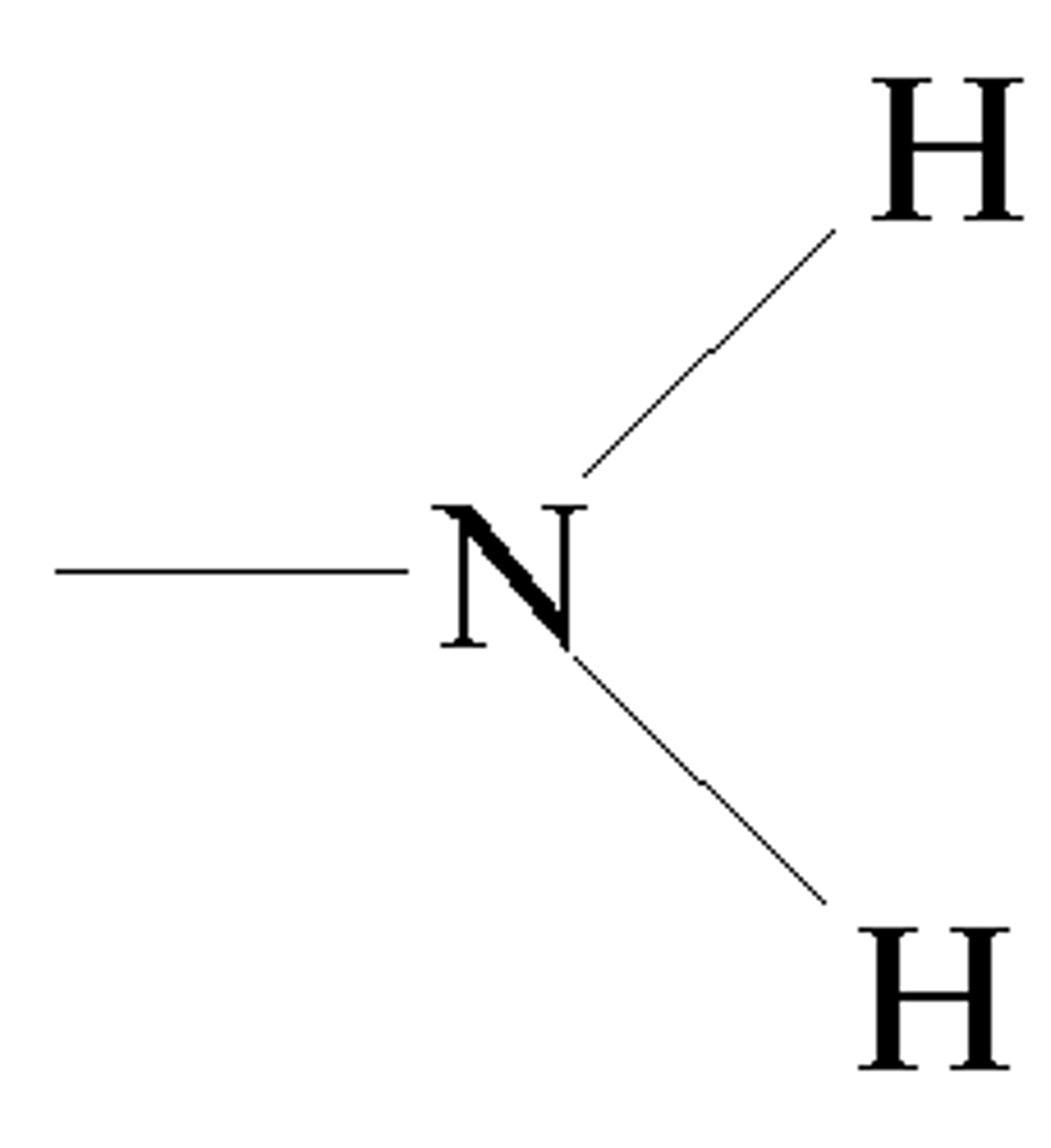
amino group
The amino group is a fundamental component of amino acids, which are the building blocks of proteins and they all share a common structure
carboxyl group
The carboxyl group is a key functional group in amino acids, which are the building blocks of proteins.
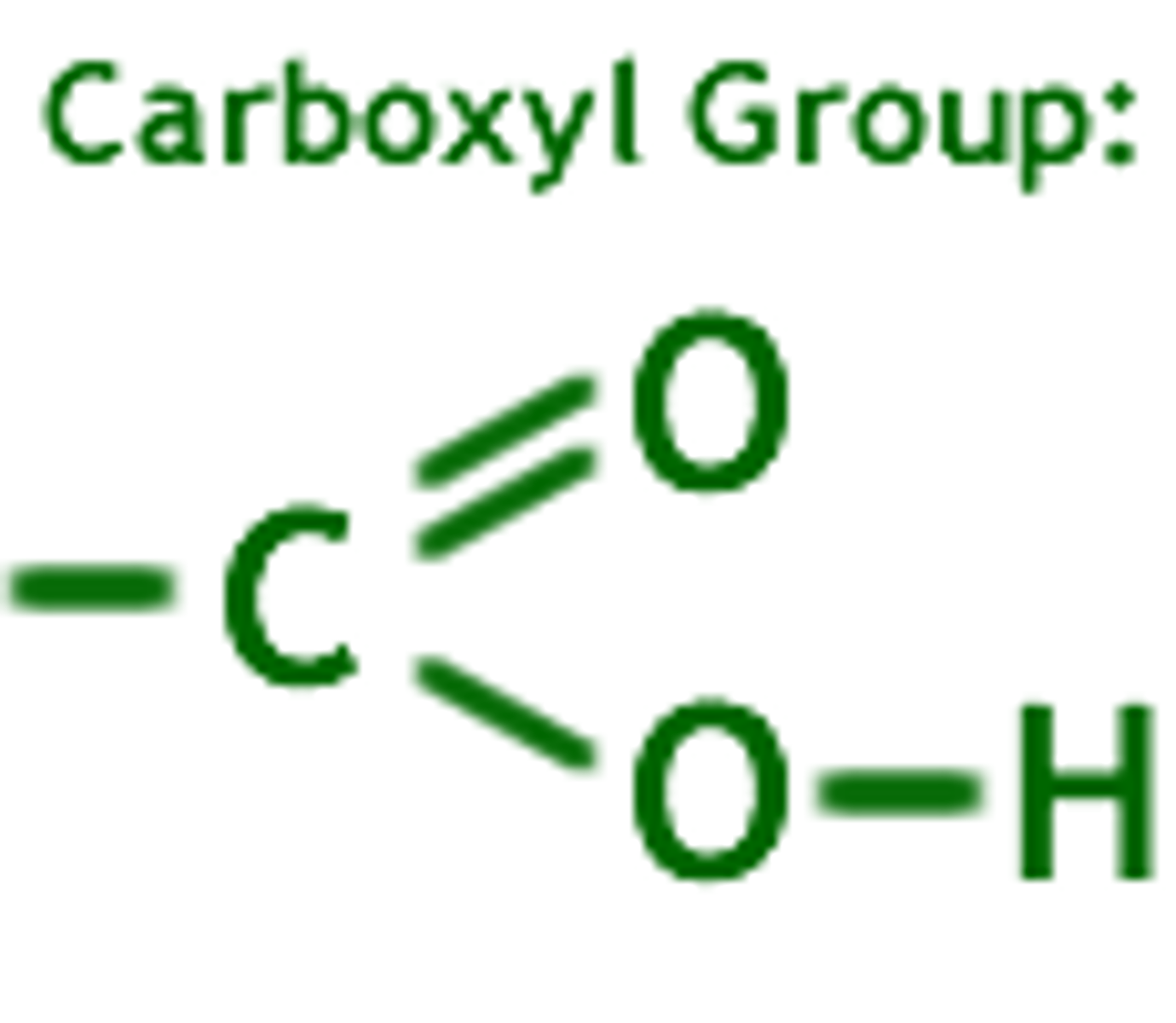
R group
The R group, or side chain, is a crucial component of amino acids, which are the building blocks of proteins.
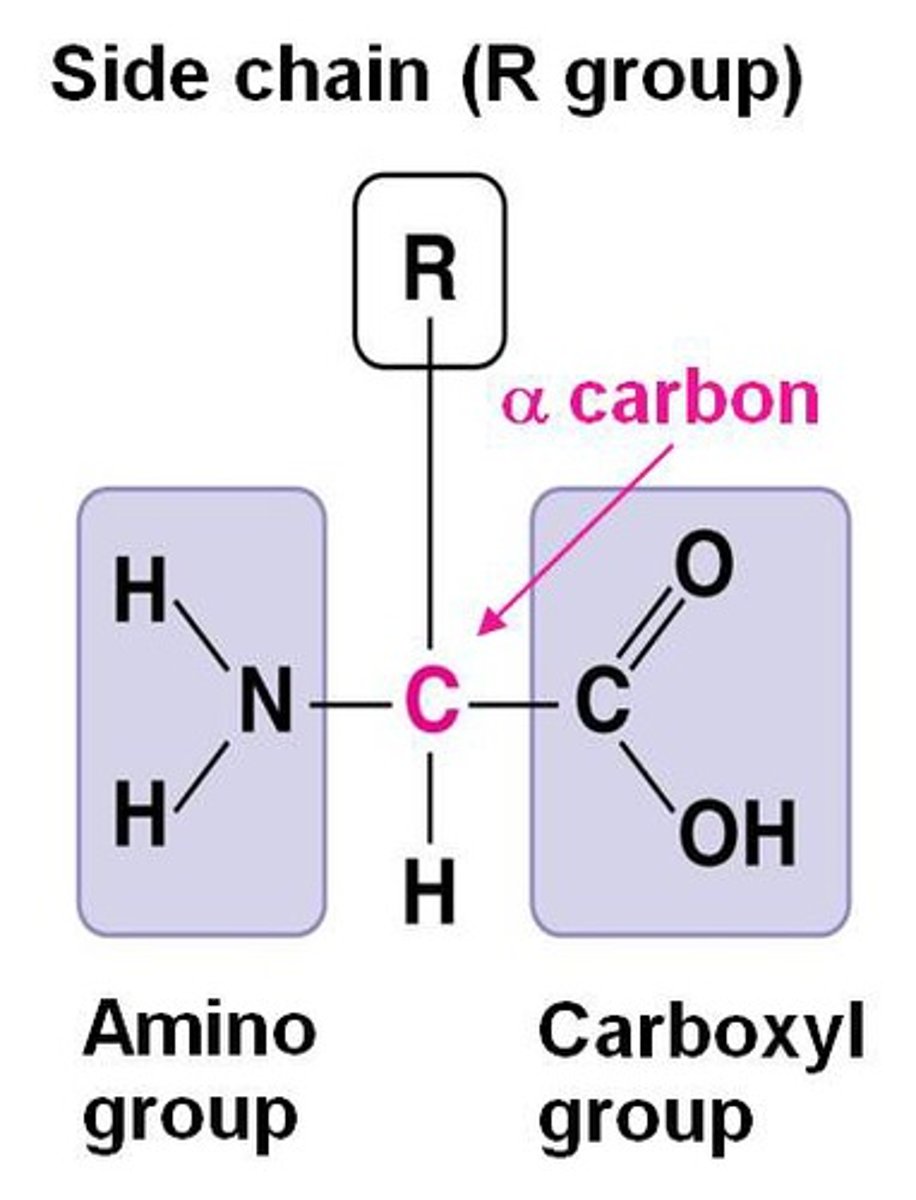
What are common side chain classifications?
Non-polar (Hydrophobic) Side Chains, Polar (Hydrophilic) Side Chains, Electrically Charged Side Chains ( Acidic (Negatively Charged), Basic (Positively Charged))
Which of these groups are involved in forming peptide bonds?
amino and carboxyl group
the basic categories of amino acids (based on R group chemistry)
Non-polar (Hydrophobic) Side Chains, Polar (Hydrophilic) Side Chains,Electrically Charged Side Chains (Hydrophilic)
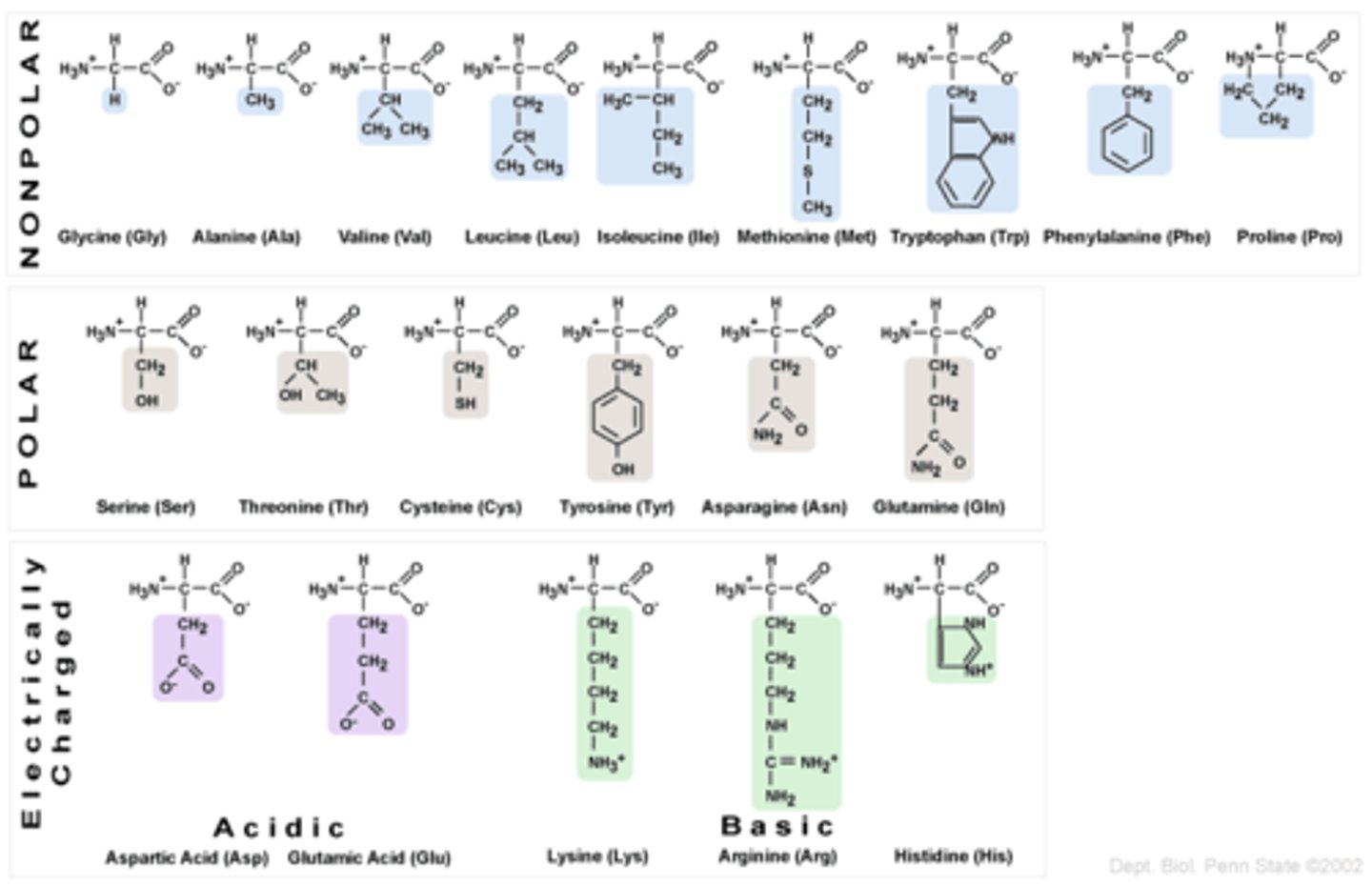
non-polar hydrophobic
Non-polar hydrophobic amino acids have side chains that do not interact well with water. These amino acids tend to cluster together within proteins, away from water, contributing to the protein's structure and stability.
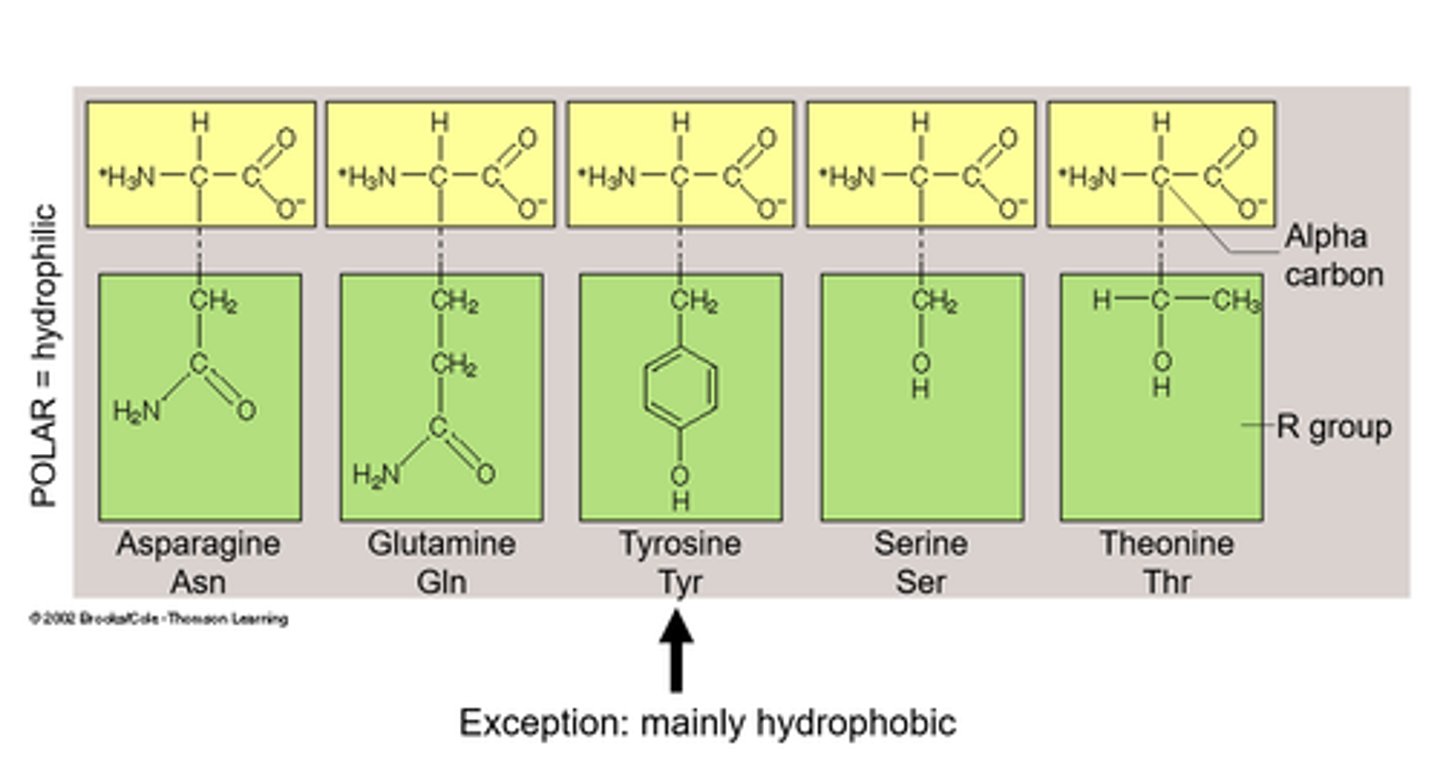
polar hydrophilic
Polar hydrophilic amino acids have side chains that can form hydrogen bonds with water, making them soluble in aqueous environments. These amino acids are crucial for protein interactions with water and other molecules.
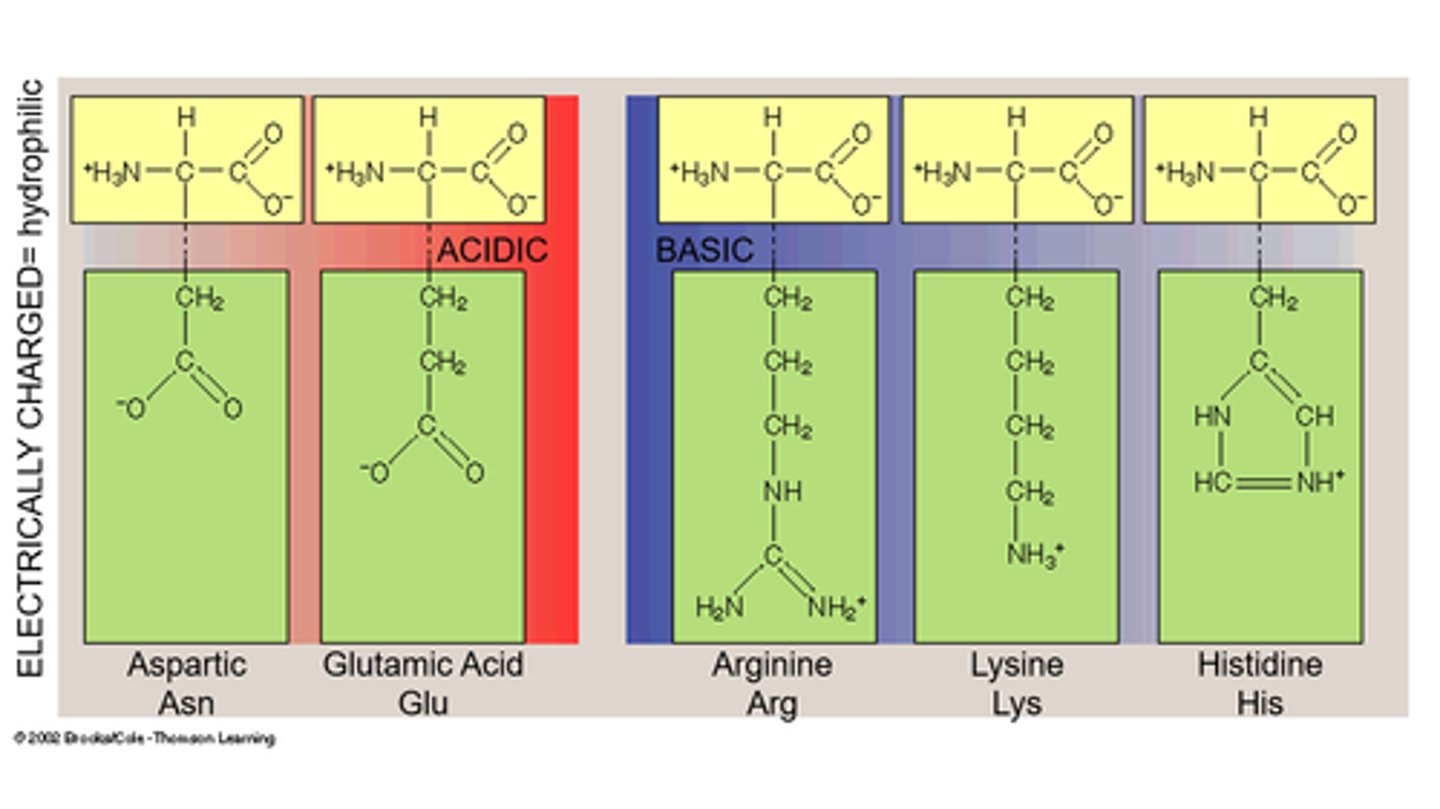
charged hydrophilic
Charged hydrophilic amino acids have side chains that carry a charge, making them attracted to water. These amino acids are essential in protein structure and function due to their ability to interact with aqueous environments.
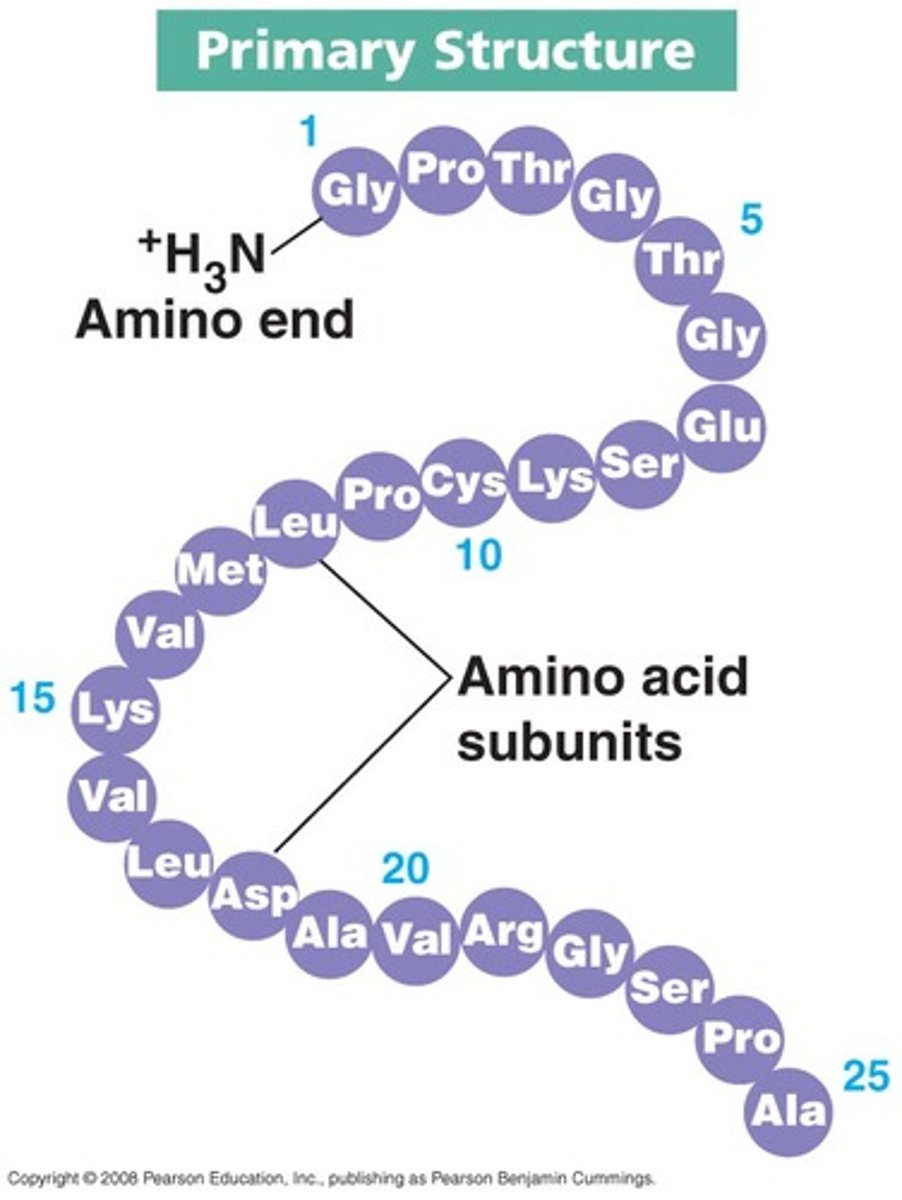
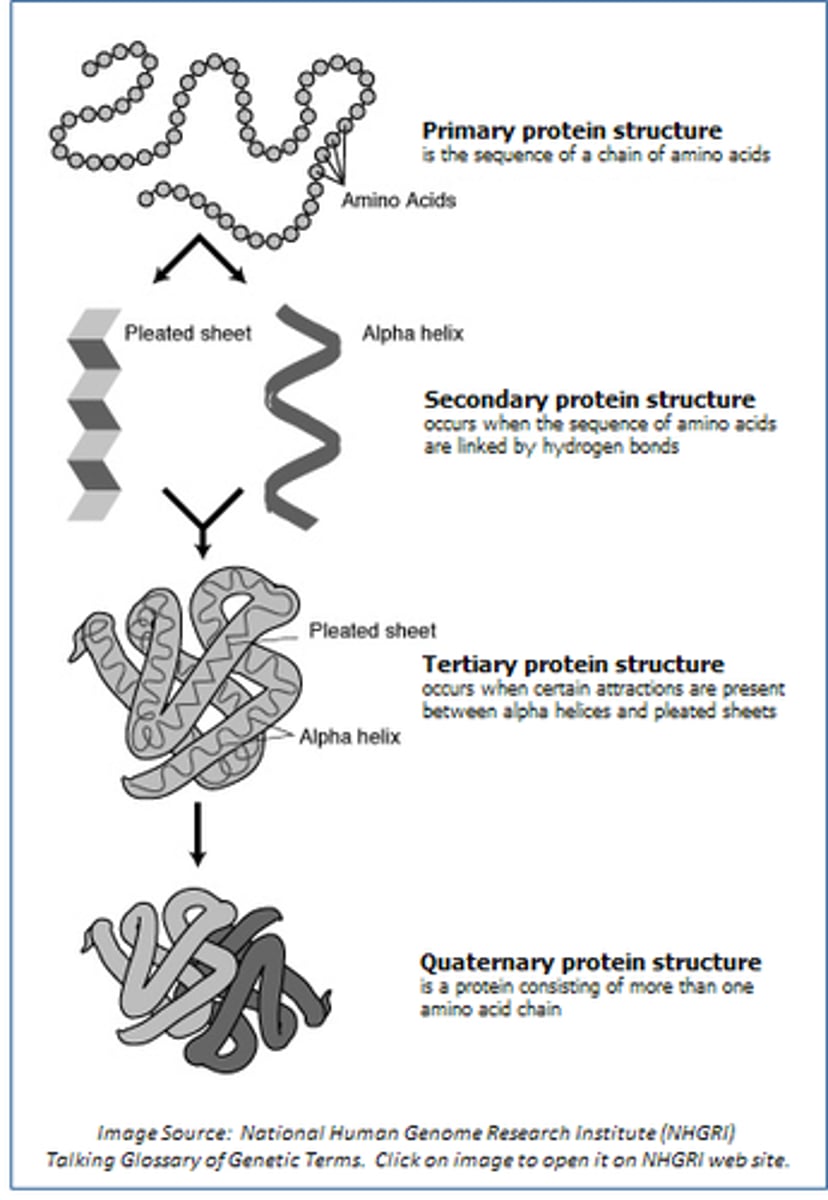
primary protein structure
The primary structure of a protein is the linear sequence of amino acids that form a polypeptide chain
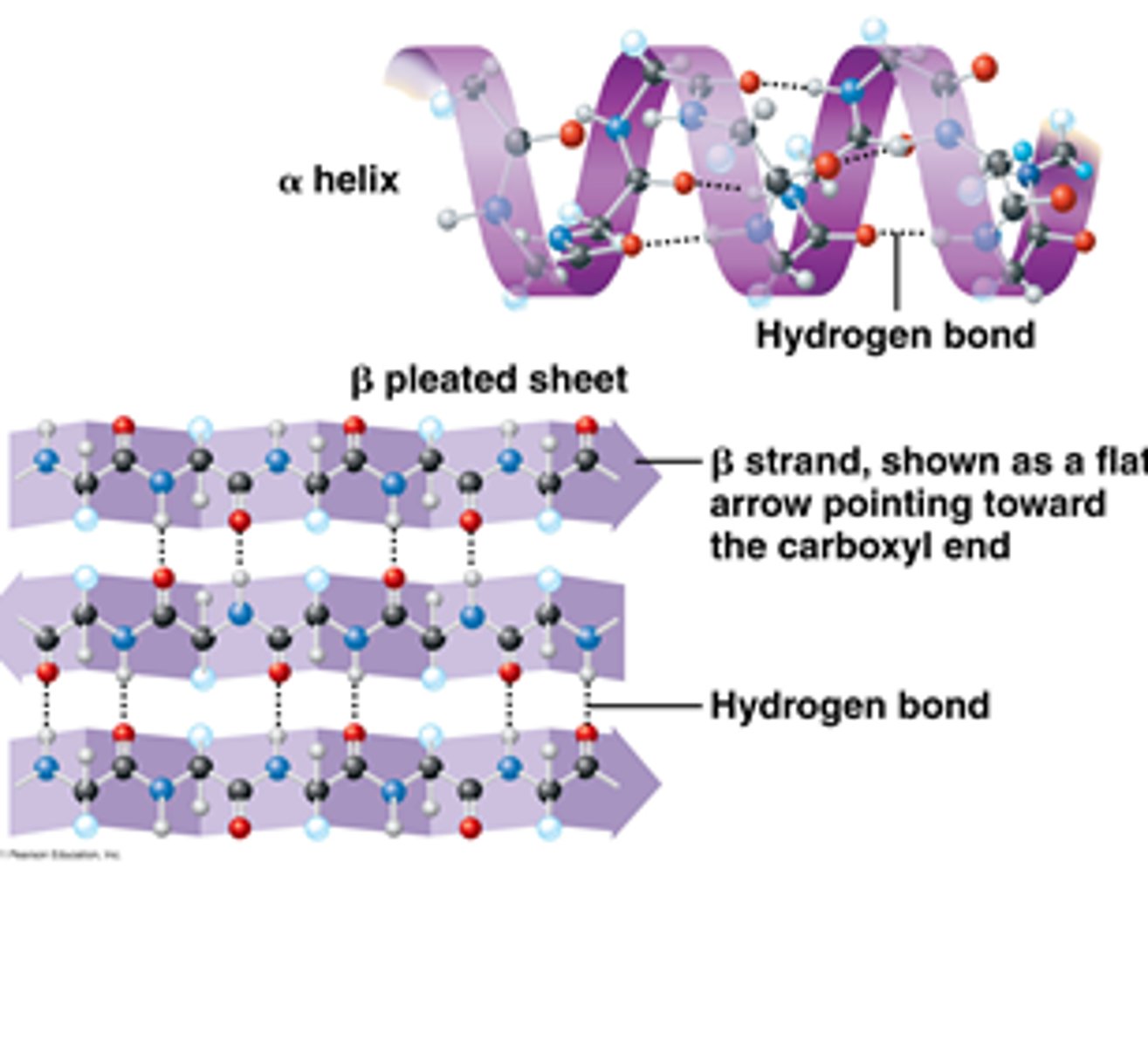
secondary protein structure
The secondary structure of a protein refers to the local folding of the polypeptide chain into specific patterns, primarily stabilized by hydrogen bonds.
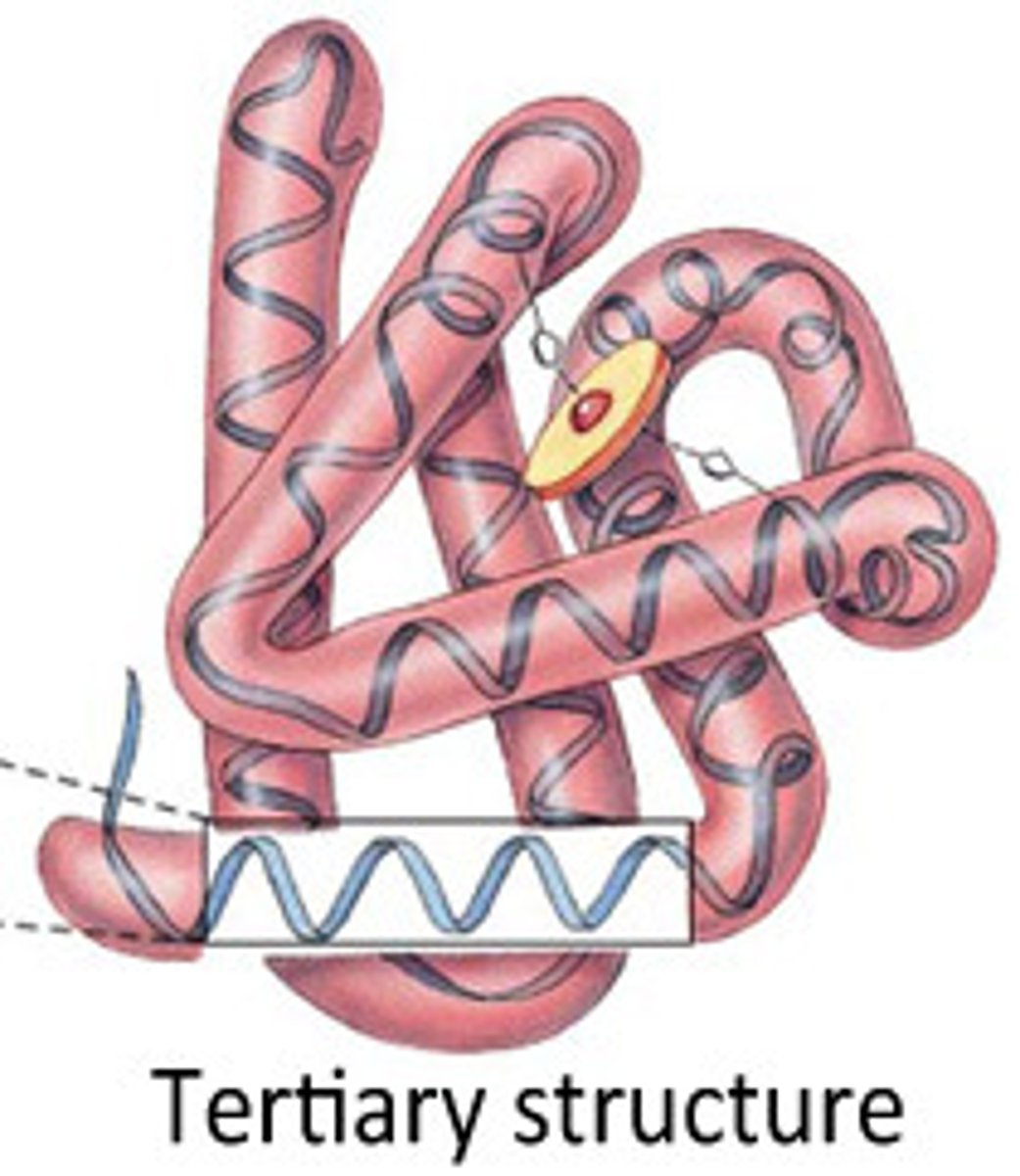
tertiary protein structure
Tertiary protein structure refers to the three-dimensional shape of a polypeptide, which is stabilized by interactions between the side chains (R groups) of amino acids
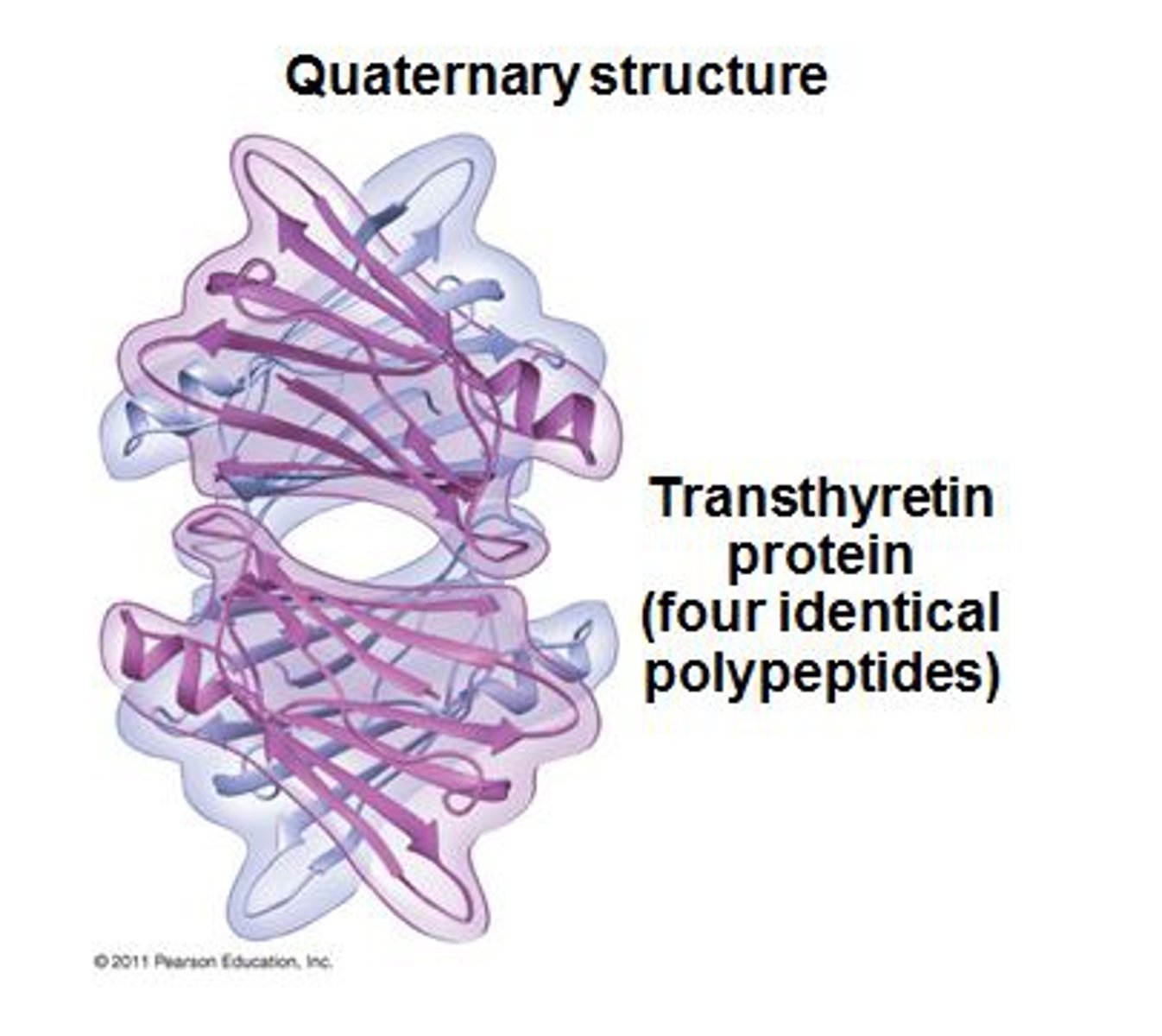
quaternary protein structure
Quaternary protein structure refers to the arrangement and interaction of multiple polypeptide chains in a protein.
What parts of the molecule are involved in each level of structure
primary: amino acids, peptide bonds
secondary: polypeptide backbone, backbone atoms, alpha-helices, beta-pleated sheets
tertiary: side chains, disulfide, hydrophobic interactions, hydrogen ionic bonds
quaternary: multiple polypeptide subunits, interactions between subunits, prosthetic groups
What types of bonds are involved in each level of structure
primary, secondary, tertiary, quaternary
Where do alpha-helices and beta-pleated sheets come into play
The sequence of amino acids determines the protein's shape—where an alpha helix can form, where beta pleated sheets can exist, where disulfide bridges are located, where ionic bonds can form, and so on.
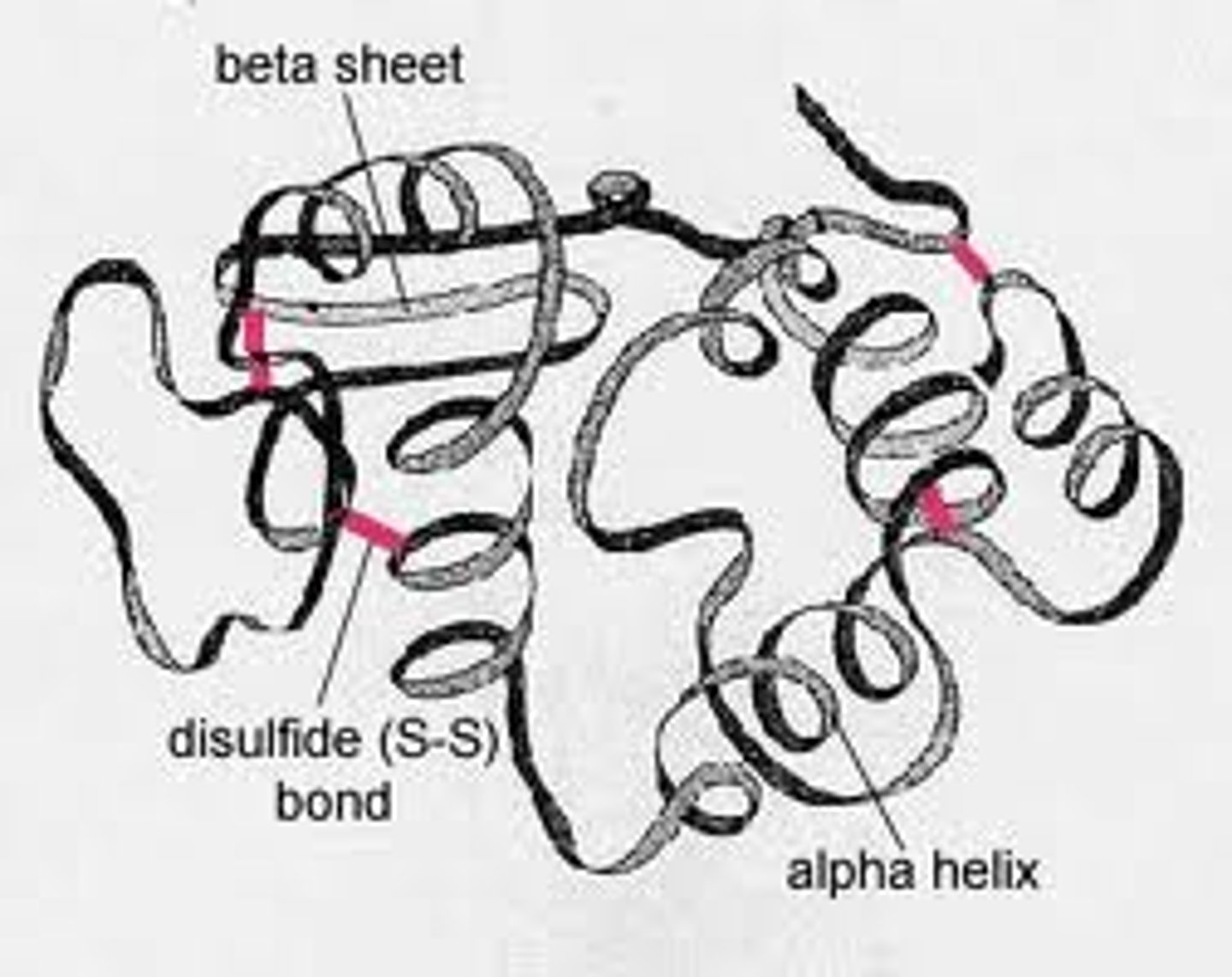
How are secondary and tertiary structure directly dependent on primary structure
The primary structure of a protein, which is its sequence of amino acids, directly influences its secondary and tertiary structures. The primary structure sets the stage for the protein's final shape and function by determining the possible interactions and folding patterns.
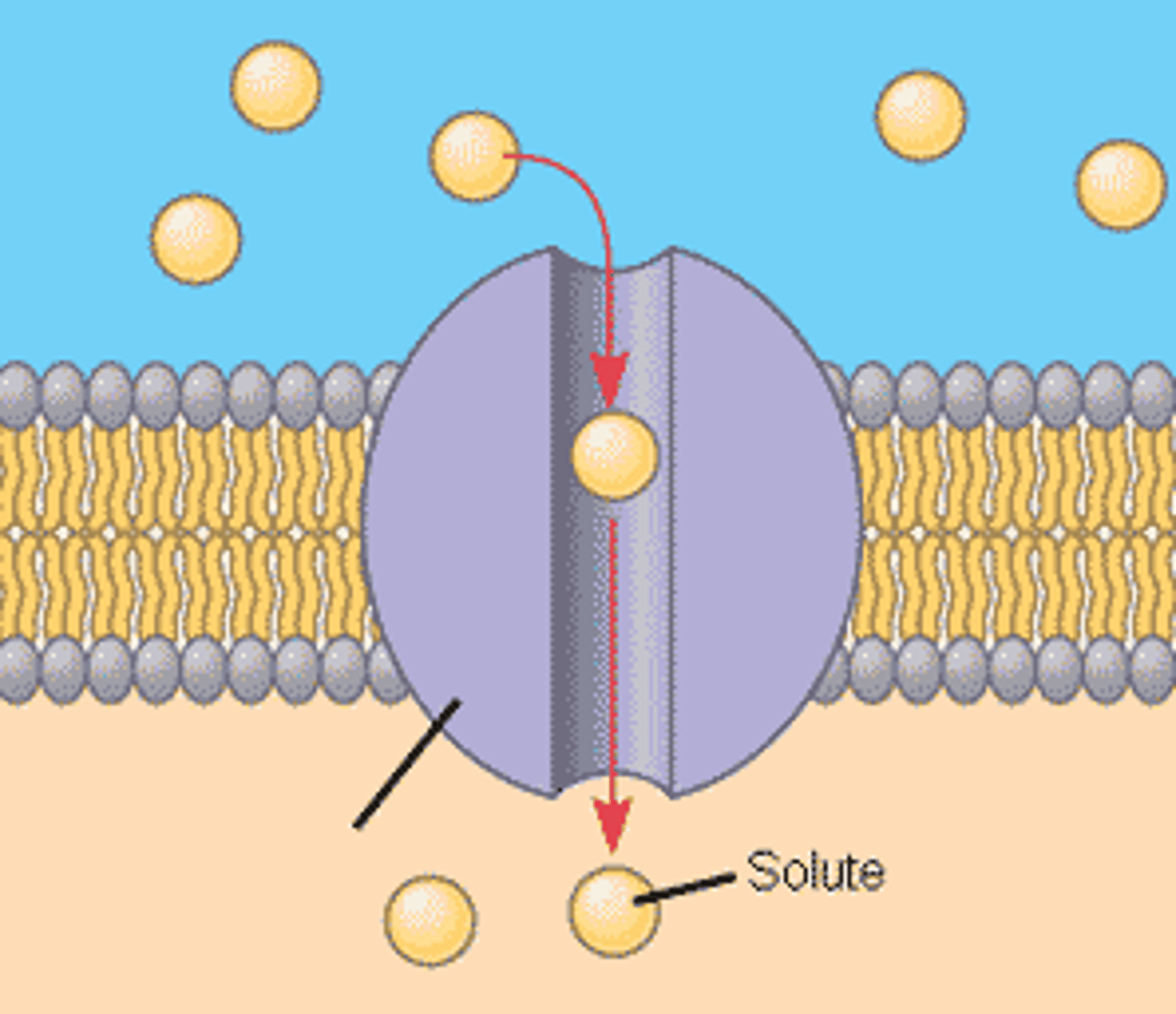
protein X
involved in the movement of molecule Zeta across the cell membrane
What kind of protein is X
Protein X is a type of protein that is destined to span the plasma membrane. This means it is likely a membrane protein, which plays a crucial role in various cellular functions