Ch 13 - Freezing-point Depression & Boiling-point Elevation
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
more solute lowers or depresses the freezing point of a solvent (bc it disrupts the orderly crystalline structure that forms when a solvent solidifies)
Remember: more solute lowers or depresses the freezing point of a solvent (bc it disrupts the orderly crystalline structure that forms when a solvent solidifies)
Change in freezing-point / freezing-point depression (∆Tf)
Def: amt by which the normal freezing-point will be lowered (NOT the new freezing-point)
Eqn: ∆Tf = Kf m
constant that is unique for each solvent
Kf or Kb
Freezing-point of the pure substance
Tf° or Tsolvent
memorize the table
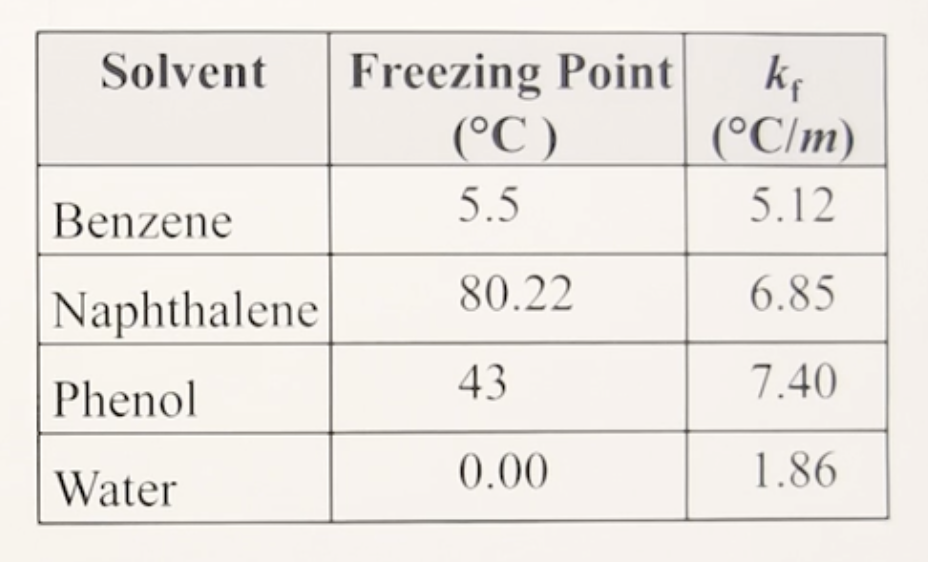
5.5 °C
Tf° of Benzene:
5.12°C/m
Kf of Benzene:
80.22 °C
Tf° of Naphthalene:
6.85°C/m
Kf of Naphthalene:
43°C
Tf° of Phenol:
7.40°C/m
Kf of Phenol:
0.00°C
Tf° of water:
1.86°C/m
Kf of water:
more solute raises or elevates the boiling point of a solvent bc it makes it harder for solvent particles to escape from the surface of the liquid
Remember: more solute raises or elevates the boiling point of a solvent bc it makes it harder for solvent particles to escape from the surface of the liquid
Change in Boiling-point / Boiling-point elevation (∆Tb)
Def: amt by which the normal boiling-point will be increased (NOT the new boiling-point)
Eqn: ∆Tb = Kb m
Boiling-point of the pure substance
Tb° or Tsolvent
memorize the table
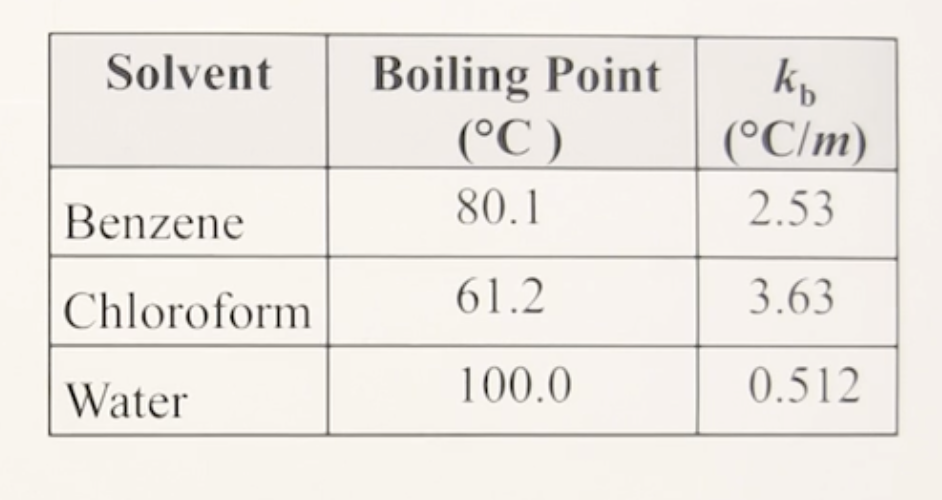
80.1°C
Tb° of Benzene:
2.53°C/m
Kb of Benzene:
61.2°C
Tb° of Chloroform:
3.63°C/m
Kb of Chloroform:
100.0°C
Tb° of Water:
0.512°C/m
Kb of Water: