pain and suffering
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
45 Terms
What is an endothermic reaction? What is delta h?
Energy is ABSORBED by the reaction, positive
What side of the equation is the endothermic number written on?
The reactants side
What is an exothermic reaction? What is delta h?
Energy is GIVEN OFF by the reaction, negative
In an exothermic reaction, what side of the equation is the number on?
The products side
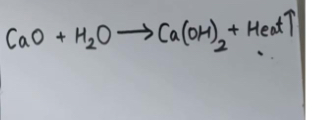
What reaction is this an example of
Exothermic

What type of reaction is this an example of
Endothermic
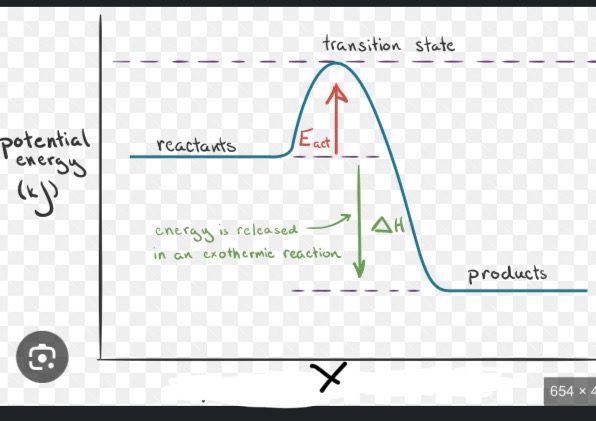
This is an exothermic reaction, what would X be labeled as?
Reaction progress
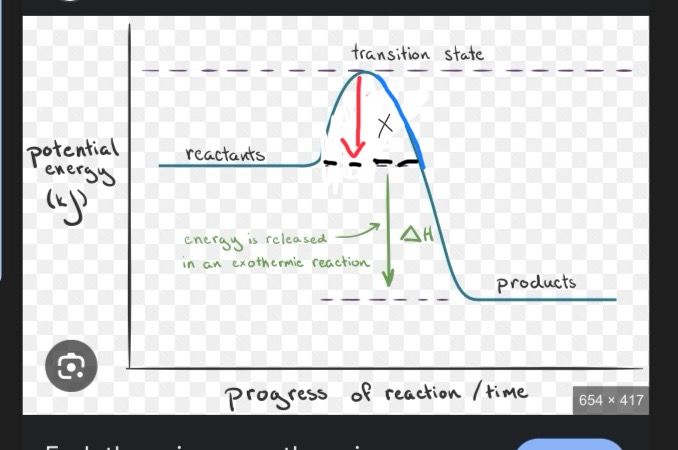
This is an exothermic reaction, what would X be labeled as?
Delta H, aka the number on the products side
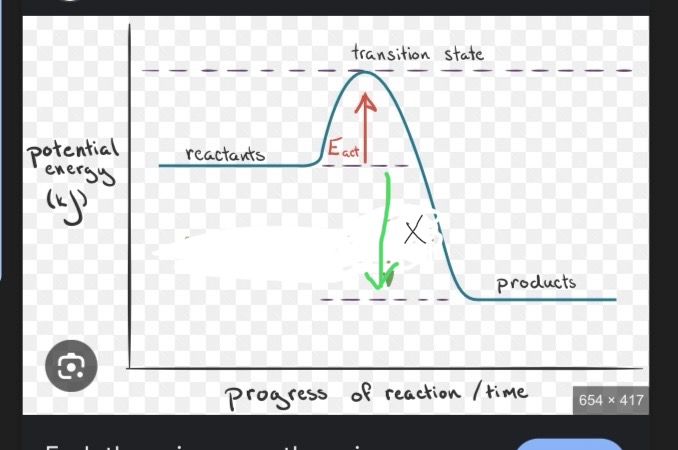
This is an exothermic reaction, what would X be labeled as?
Ea
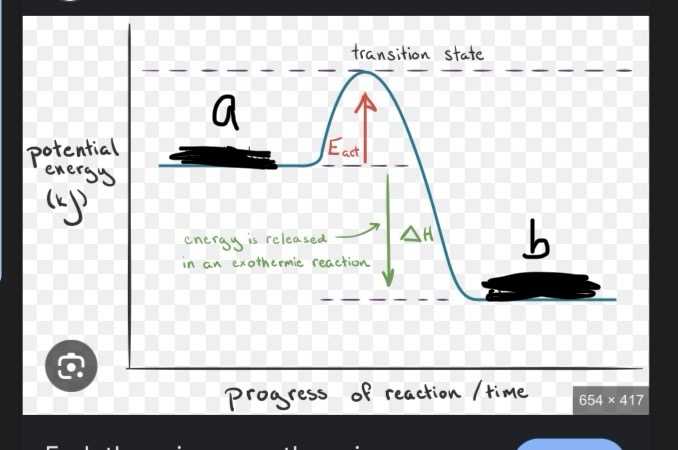
This is an exothermic reaction, what would a an and b be labeled as?
a= reactants b=products
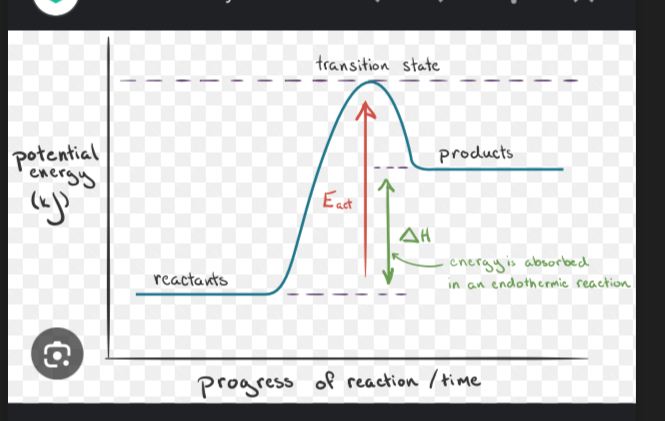
What type of energy diagram is this portraying
Endothermic
On an exothermic energy diagram, the reactants are _____ the products
Above
On an endothermic energy diagram, the reactants are _____ the products
Below
On an energy diagram, which is listed first?
Reactants
Where do you draw the ea on a energy diagram?
From the reactant line to the top of the arch
Where do you label delta h on an energy diagram?
The reactants line to the products line
If a catalyst is added to a reaction on an energy diagram, how would that change the diagram?
The arch would be smaller
Speeds up a chem reaction by lowering the activation energy (ea)
Catalyst/enzyme
Delta H Doesn’t affect the rate of the chemical reaction, but generally exothermic reactions are ___ than endothermic reactions
Faster
Define what rate of reaction means
How fast (increased rate) or slow (decreased rate) a reaction goes
What factors affect the rate of reaction
The concentration of each reactant, temperature, or if a catalyst is added
How does concentration affect the rate of reaction
The higher the concentration, the faster the reaction
How does temp affect rate of reaction
Higher the temp, faster the reaction
How does a catalyst affect the rate of reaction
Increases the rate
How to tell if a molecule is a trigonal pyramidal or a trigonal planar
Trigonal pyramidal have a lone electron pair
Prefix for 1
Mono
Prefix for 2
Di
Prefix for e
Tri
Prefix for 4
Tetra
Prefix for 5
Penta
Prefix for 6
Hexa
Prefix for 7
Hepta
Prefix for 8
Octa
Prefix fir 9
Nona
Prefix for 10
Deca
How to tell electronegativity from the periodic table
Gets smaller as it goes left and down
What makes a carbon pairing polar
If the element is more electronegative than carbon
What makes a carbon pairing non polar
If the connected element has a lesser electronegativity
Most common polar pairing with another element bc of high electronegativity
F, N, O, or any halogen
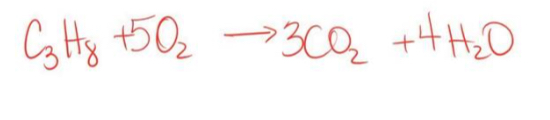
What type of reaction is this? How can you tell?
Combustion bc a CO2 and H2O were formed
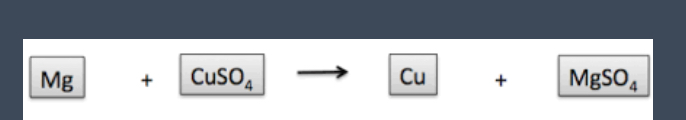
What type of reaction is this
Single displacement

What type of reaction is this
Double displacement
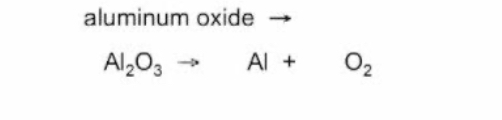
What type of reaction is this
Decomposition
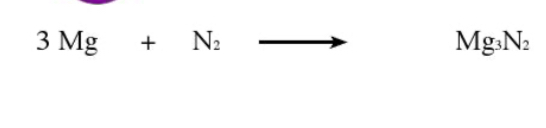
What type of reaction is this
Combination/synthesis
When doing stoichiometry, how do you determine the mol to mol ration
By using the prefix numbers in the equation