chem Topic 6 kinetics (copy)
1/10
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
What are the two things for a successful collision?
Particles colliding must have kinetic energies equal to or grater than the reaction’s activation energy.
The particle must collide with the correct geometry/orientation
What is the activation energy and what does it represent?
The activation energy is defined as the minimum value of kinetic energy which particles must have to react.
It represents an “energy barrier” for the reaction.
What are the factors affecting rates of reaction
Temperature (only one that does it in 2 ways)
Catalyst
Conentration
Surface area (particle size)
Pressure (reactions involving gases)
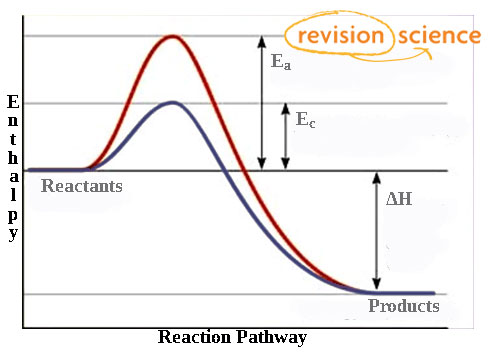
How do catalysts work and why do they increase rate of reaction.
They provide an alternate reaction pathway that has a lower activation energy.
Catalysts increase the number and proportion of particles that have sufficient energy to meet or exceed activation energy.
∴ increase freuency and proportion of successful collisions
∴ increase ROR
How does increasing temperature affect rate of reaction?
Increasing the temperature increases the proportion of reactant particles with sufficient energy to meet or exceed the reactions activation energy. As well as increases the average random kinetic energy of particles.
∴ increase freuency and proportion of successful collisions
∴ increase ROR
How does increasing concentration affect rate of reaction?
As concentration increases there are more particles available to react in a given volume.
∴ increase freuency of successful collisions
∴ increase ROR
How does decreasing particle size affect rate of reaction?
decreasing particle size increases total surface area
∴ increase in number of particles available to react
∴increase in number and frequency of successful collisions
∴ increase ROR
How does increasing pressure increase the rate of reaction in a reaction involving gases?
as pressure increases the gas is compressed and so the concentration increases.
As concentration increases there are more particles available to react.
∴ increase freuency of successful collisions
∴ increase ROR
Catalysts and enthalpy level diagrams
equal redutoin in the activation energy of both forward and backwards reactions
hump is lowered
rate of reaction
is expressed by change in concentration of a particular reactant/product per unit time
this can be indirectly observed through changes in mass, volume and color.
Why does rate of reaction plateua or decrease over time
less frequency/chance of collisions as reaction proceeds