Chemistry Paper 1
0.0(0)
Card Sorting
1/189
Earn XP
Description and Tags
Last updated 5:28 PM on 5/21/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
190 Terms
1
New cards
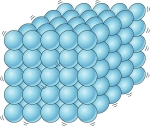
1\.1 What are the properties of a solid?
* Close, regular arrangement
* Vibrate about a fixed position
* Very low kinetic energy
* Very strong forces of attraction
* Vibrate about a fixed position
* Very low kinetic energy
* Very strong forces of attraction
2
New cards
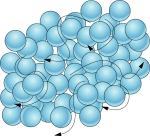
1\.1 What are the properties of a liquid?
* Close, random arrangement
* Move around each other
* Low kinetic energy
* Strong forces of attraction
* Move around each other
* Low kinetic energy
* Strong forces of attraction
3
New cards
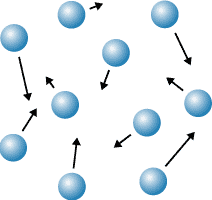
1\.1 What are the properties of a gas?
* Far, random arrangement
* Move quickly in all directions
* High kinetic energy
* Very weak forces of attraction
* Move quickly in all directions
* High kinetic energy
* Very weak forces of attraction
4
New cards
1\.2 What is melting?
When a solid changes into a liquid, requiring heat energy which transforms into kinetic energy, particles move at a specific temperature known as the melting point which is unique to each pure solid
5
New cards
1\.2 What is vaporising?
When a liquid changes into a gas, occurring only at the surface of liquids where high energy particles can escape from the liquids surface at low temperatures and below the boiling point of the liquid over a range of temperatures, heating speeds up the process as particles need energy to escape from the surface
The larger the surface area and the warmer the liquid/surface, the more quickly a liquid can evaporate
The larger the surface area and the warmer the liquid/surface, the more quickly a liquid can evaporate
6
New cards
1\.2 What is condensing?
When a gas changes into a liquid, usually on cooling as its particles lose energy and when they bump into each other, they lack energy to bounce away again, instead grouping together to form a liquid
7
New cards
1\.2 What is freezing?
When a liquid changes into a solid, occurring at exactly the same temperature as melting, hence the melting point and freezing point of a pure substance are the same, it requires a significant decrease in temperature (or loss of thermal energy) and occurs at a specific temperature which is unique for each pure substance
8
New cards
1\.2 What is sublimation?
When a solid changes directly into a gas, happening to only a few solids, such as iodine or solid carbon dioxide
9
New cards
1\.2 What is deposition?
When a gas changes directly into a solid, happening to only a few solids, such as iodine or solid carbon dioxide
10
New cards
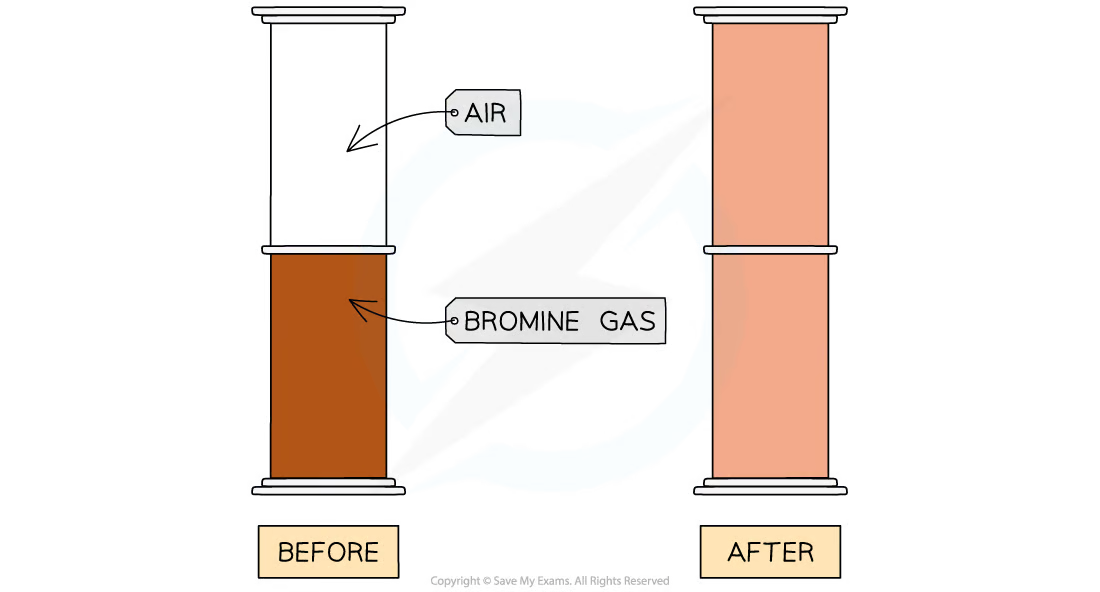
1\.3 Why do we see the diffusion of bromine gas from one gas jar to another?
The air and bromine particles are moving randomly and there are large gaps between particles so the particles can therefore easily mix together
11
New cards
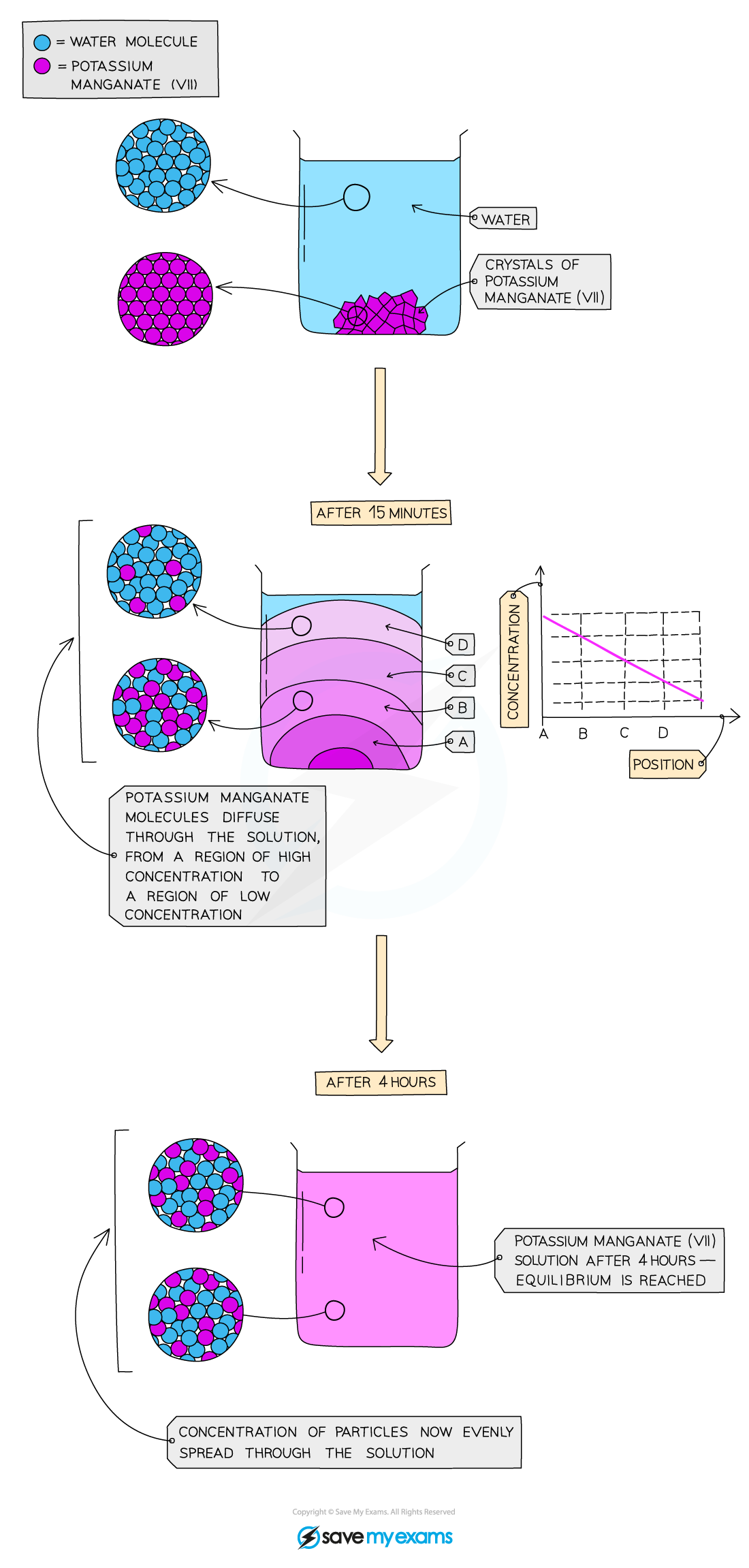
1\.3 Why does dissolving potassium manganate (VII) crystals in water form a purple solution?
The water and potassium manganate (VII) particles are moving randomly and the particles can slide over each other so the particles can therefore easily mix together but diffusion in liquids is slower than in gases because the particles in a liquid are closely packed together and move more slowly
12
New cards
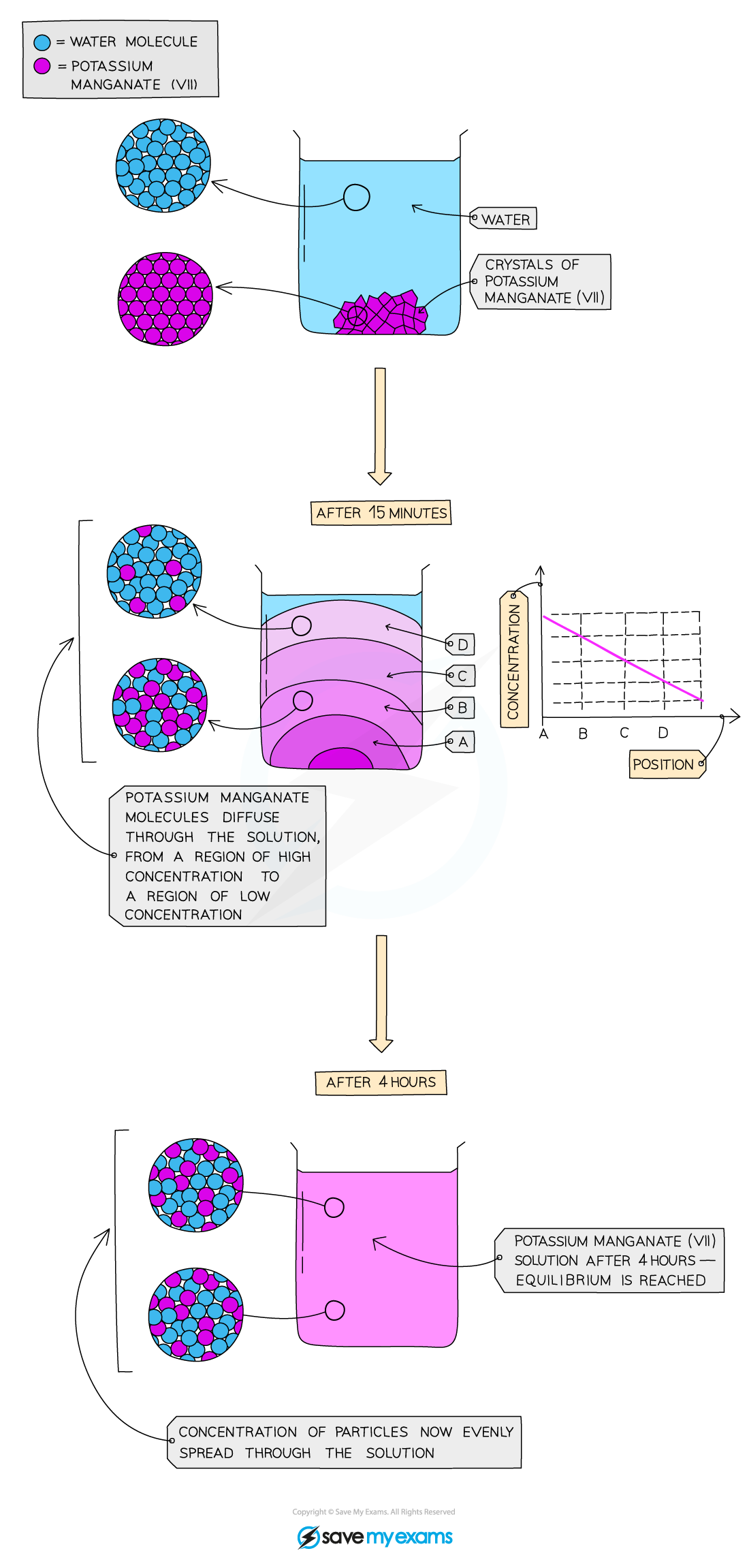
1\.3 Why does dissolving potassium magnate (VII) crystals in water several times make the colour fade but does not disappear until a lot of dilutions have been done?
There are a lot of particles in a small amount of potassium manganate (VII) and therefore the particles must be very small
13
New cards
1\.4 What is a solvent?
The liquid in which a solute dissolves
14
New cards
1\.4 What is a solute?
The substance which dissolves in a solvent to form a solution
15
New cards
1\.4 What is a solution?
The mixture formed when a solute dissolves in a solvent
16
New cards
1\.4 What is a saturated solution?
A solution with the maximum concentration of solute dissolved in the solvent
17
New cards
1\.8 What is an element?
A substance made up of atoms that all contain the same number of protons and cannot be split into anything simpler
18
New cards
1\.8 What is a compound?
A pure substance made up of two or more elements chemically bonded together
19
New cards
1\.8 What is a mixture?
A combination of two or more elements that are not chemically bonded together
20
New cards
1\.9 What is the difference between a pure substance and a mixture?
A pure substance has a fixed melting and boiling point but a mixture may melt or boil over a range of temperatures
21
New cards
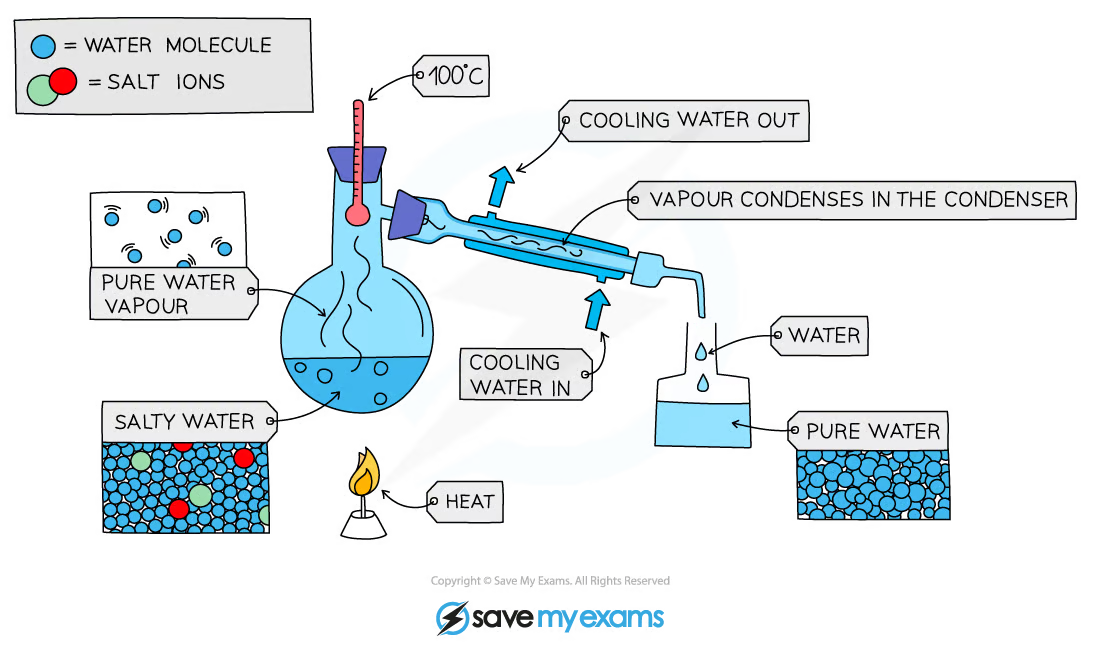
1\.10 How do you separate a mixture using simple distillation?
* Used to separate a liquid and soluble solid from a solution or a pure liquid from a mixture of liquids
* The solution is heated, and pure water evaporates producing a vapour which rises through the neck of the round-bottomed flask
* The vapour passes through the condenser, where it cools and condenses, turning into the pure liquid that is collected in a beaker
* After all the water is evaporated from the solution, only the solid solute will be left behind
* The solution is heated, and pure water evaporates producing a vapour which rises through the neck of the round-bottomed flask
* The vapour passes through the condenser, where it cools and condenses, turning into the pure liquid that is collected in a beaker
* After all the water is evaporated from the solution, only the solid solute will be left behind
22
New cards
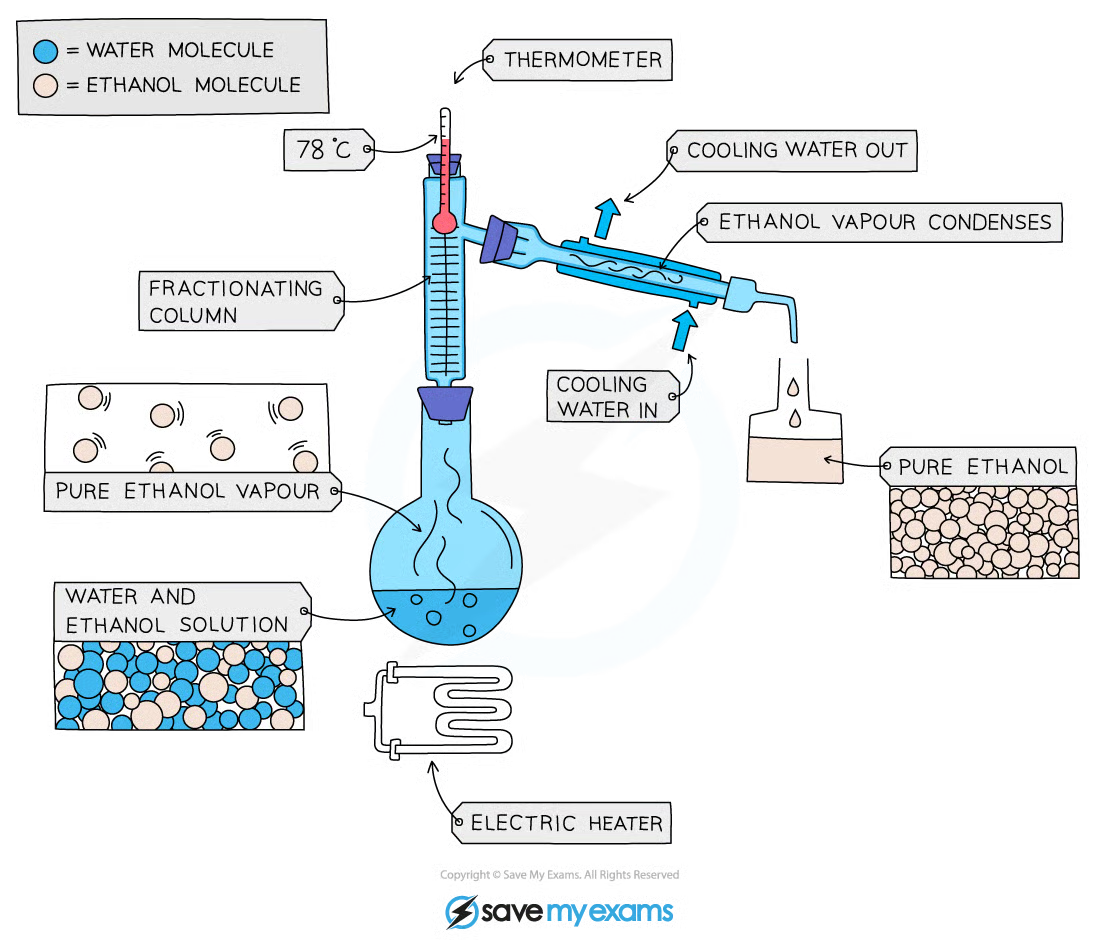
1\.10 How do you separate a mixture using fractional distillation?
* This is used to separate two or more liquids that are **miscible** from one another
* The solution is heated to the temperature of the substance with the lowest boiling point
* This substance will rise and evaporate first, and vapours will pass through a condenser, where they cool and condense, turning into a liquid that will be collected in a beaker
* All of the substance is evaporated and collected, leaving behind the other components(s) of the mixture
* For water and ethanol
* Ethanol has a boiling point of 78 ºC and water of 100 ºC
* The mixture is heated until it reaches 78 ºC, at which point the ethanol boils and distils out of the mixture and condenses into the beaker
* When the temperature starts to increase to 100 ºC heating should be stopped. Water and ethanol are now separated
* The solution is heated to the temperature of the substance with the lowest boiling point
* This substance will rise and evaporate first, and vapours will pass through a condenser, where they cool and condense, turning into a liquid that will be collected in a beaker
* All of the substance is evaporated and collected, leaving behind the other components(s) of the mixture
* For water and ethanol
* Ethanol has a boiling point of 78 ºC and water of 100 ºC
* The mixture is heated until it reaches 78 ºC, at which point the ethanol boils and distils out of the mixture and condenses into the beaker
* When the temperature starts to increase to 100 ºC heating should be stopped. Water and ethanol are now separated
23
New cards
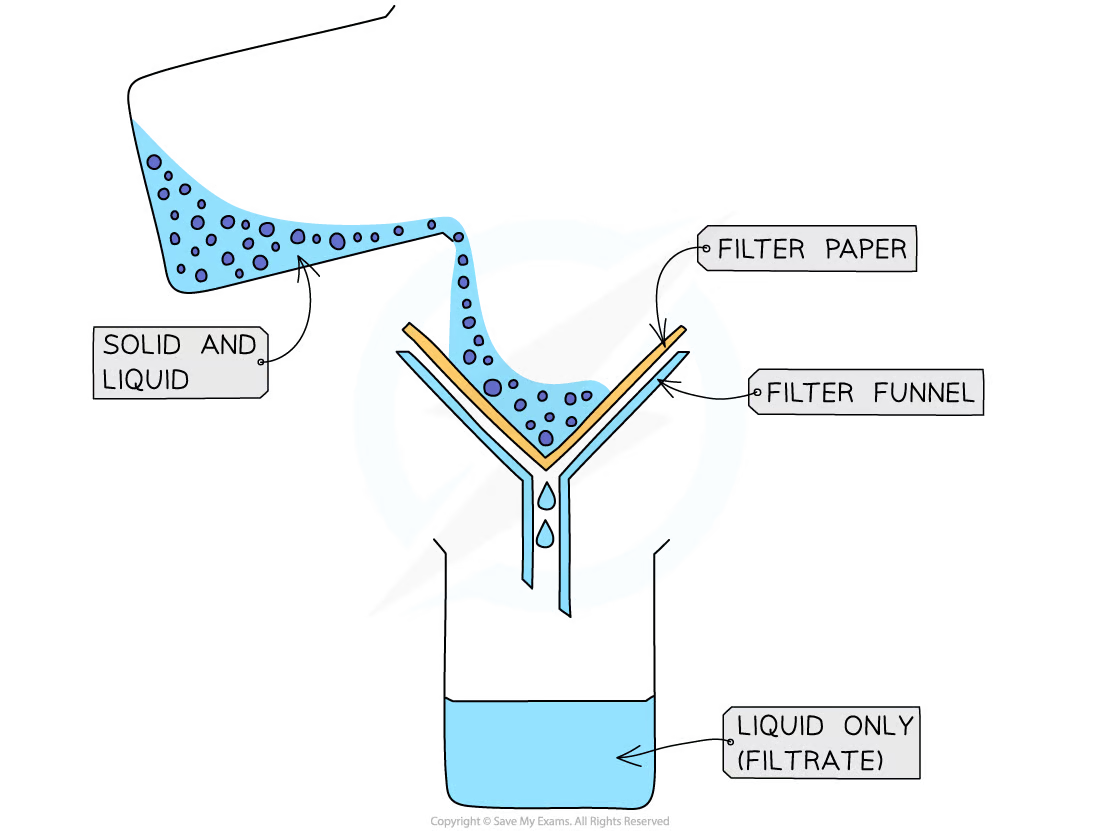
1\.10 How do you separate a mixture using filtration?
* Used to separate an undissolved solid from a mixture of the solid and a liquid/solution
* A piece of filter paper is placed in a filter funnel above a beaker
* A mixture of insoluble solid and liquid is poured into the filter funnel
* The filter paper will only allow small liquid particles to pass through as filtrate
* Solid particles are too large to pass through the filter paper so will stay behind as a residue
* A piece of filter paper is placed in a filter funnel above a beaker
* A mixture of insoluble solid and liquid is poured into the filter funnel
* The filter paper will only allow small liquid particles to pass through as filtrate
* Solid particles are too large to pass through the filter paper so will stay behind as a residue
24
New cards
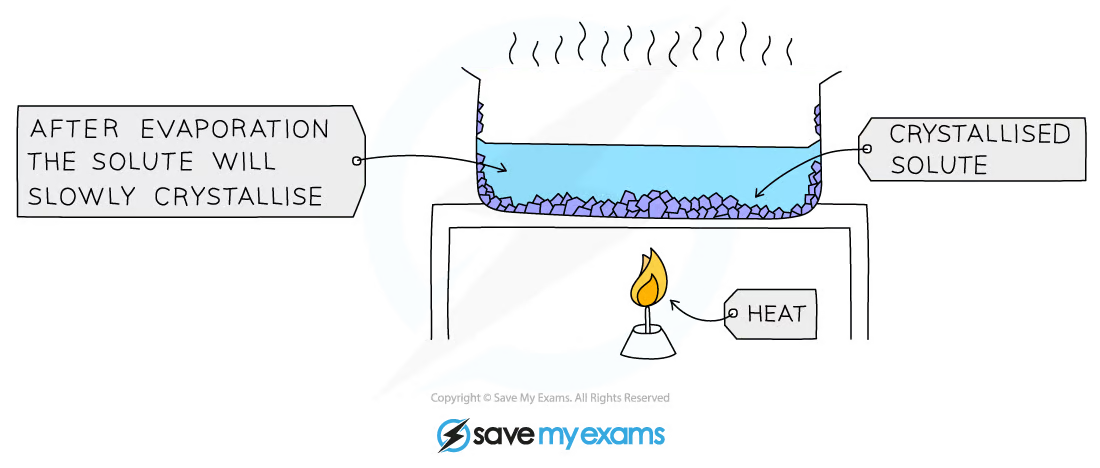
1\.10 How do you separate a mixture using crystallisation?
* Used to separate a dissolved solid from a solution, when the solid is much more soluble in hot solvent than in cold (e.g., copper sulphate from a solution of copper (II) sulphate in water)
* The solution is heated, allowing the solvent to evaporate, leaving a saturated solution behind
* Test if the solution is saturated by dipping a clean, dry, cold glass rod into the solution and if the solution is saturated, crystals will form on the glass rod
* The saturated solution is allowed to cool slowly
* Crystals begin to grow as solids will come out of solution due to decreasing solubility
* The crystals are collected by filtering the solution, they are washed with cold distilled water to remove impurities and are then allowed to dry
* The solution is heated, allowing the solvent to evaporate, leaving a saturated solution behind
* Test if the solution is saturated by dipping a clean, dry, cold glass rod into the solution and if the solution is saturated, crystals will form on the glass rod
* The saturated solution is allowed to cool slowly
* Crystals begin to grow as solids will come out of solution due to decreasing solubility
* The crystals are collected by filtering the solution, they are washed with cold distilled water to remove impurities and are then allowed to dry
25
New cards
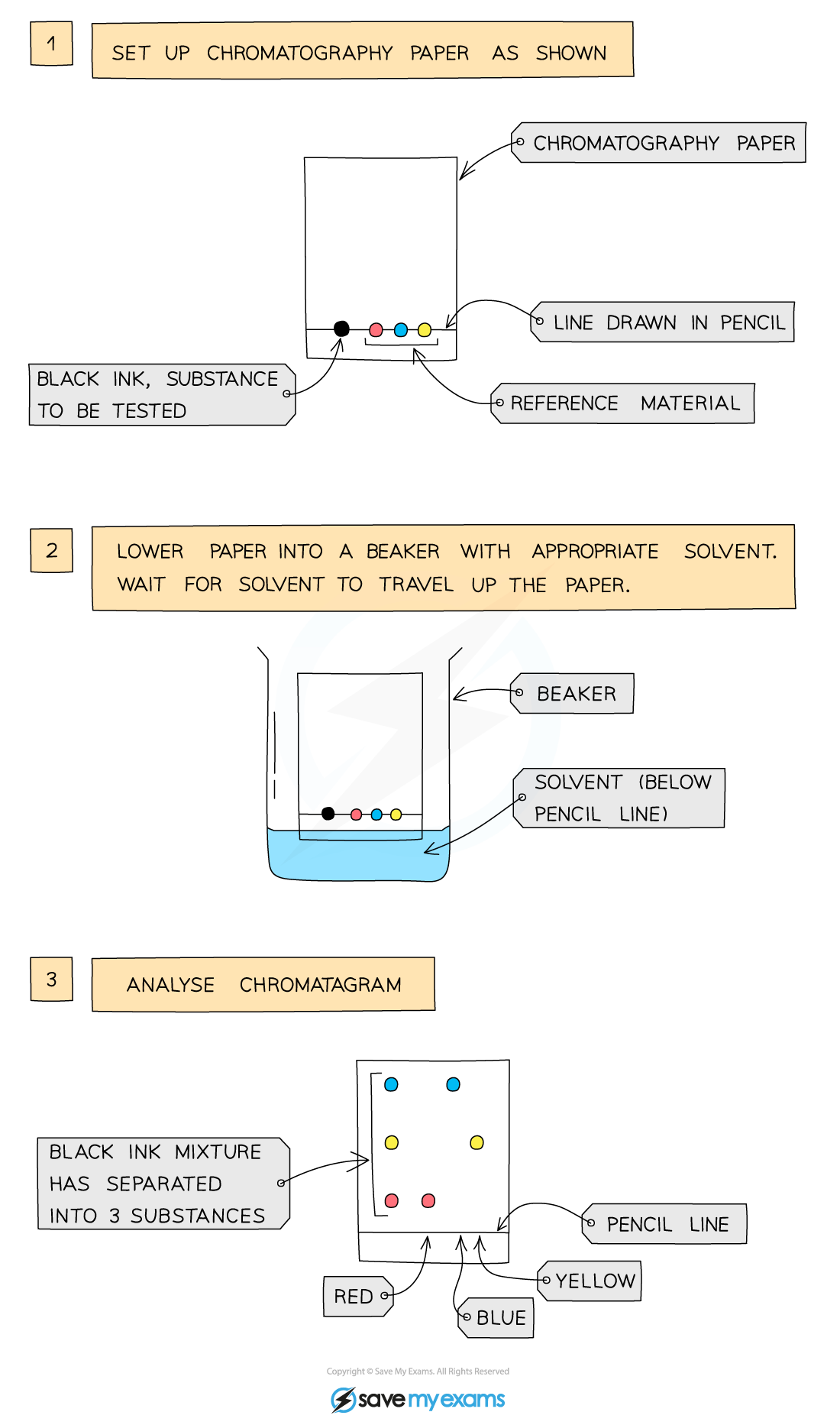
1\.10 How do you separate a mixture using paper chromatography?
* This technique is used to separate substances that have different solubilities in a given solvent (e.g., different coloured inks that have been mixed to make black ink)
* A pencil line is drawn on chromatography paper and spots of the sample are placed on it. Pencil is used for this as ink would run into the chromatogram along with the samples
* The paper is then lowered into the solvent container, making sure that the pencil line sits above the level of the solvent, so the samples don’t wash into the solvent container
* The solvent travels up the paper by capillary action, taking some of the coloured substances with it; it is called the mobile phase
* Different substances have different solubilities so will travel at different rates, causing the substances to spread apart and the substances with higher solubility will travel further than the others
* This will show the different components of the ink/dye
* If two or more substances are the same, they will produce identical chromatograms
* If the substance is a mixture, it will separate on the paper to show all the different components as separate spots
* An impure substance will show up with more than one spot, a pure substance should only show up with one spot
* A pencil line is drawn on chromatography paper and spots of the sample are placed on it. Pencil is used for this as ink would run into the chromatogram along with the samples
* The paper is then lowered into the solvent container, making sure that the pencil line sits above the level of the solvent, so the samples don’t wash into the solvent container
* The solvent travels up the paper by capillary action, taking some of the coloured substances with it; it is called the mobile phase
* Different substances have different solubilities so will travel at different rates, causing the substances to spread apart and the substances with higher solubility will travel further than the others
* This will show the different components of the ink/dye
* If two or more substances are the same, they will produce identical chromatograms
* If the substance is a mixture, it will separate on the paper to show all the different components as separate spots
* An impure substance will show up with more than one spot, a pure substance should only show up with one spot
26
New cards
1\.11 How does a chromatogram provides information about the composition of a mixture?
* Pure substances will produce only one spot on the chromatogram
* If two or more substances are the same, they will produce identical chromatograms
* If the substance is a mixture, it will separate on the paper to show all the different components as separate spots
* If two or more substances are the same, they will produce identical chromatograms
* If the substance is a mixture, it will separate on the paper to show all the different components as separate spots
27
New cards
1\.12 How do you use the calculation of Rf values to identify the components of a mixture?
Rf = distance travelled by substance ÷ distance travelled by solvent (both measured from baseline)
28
New cards
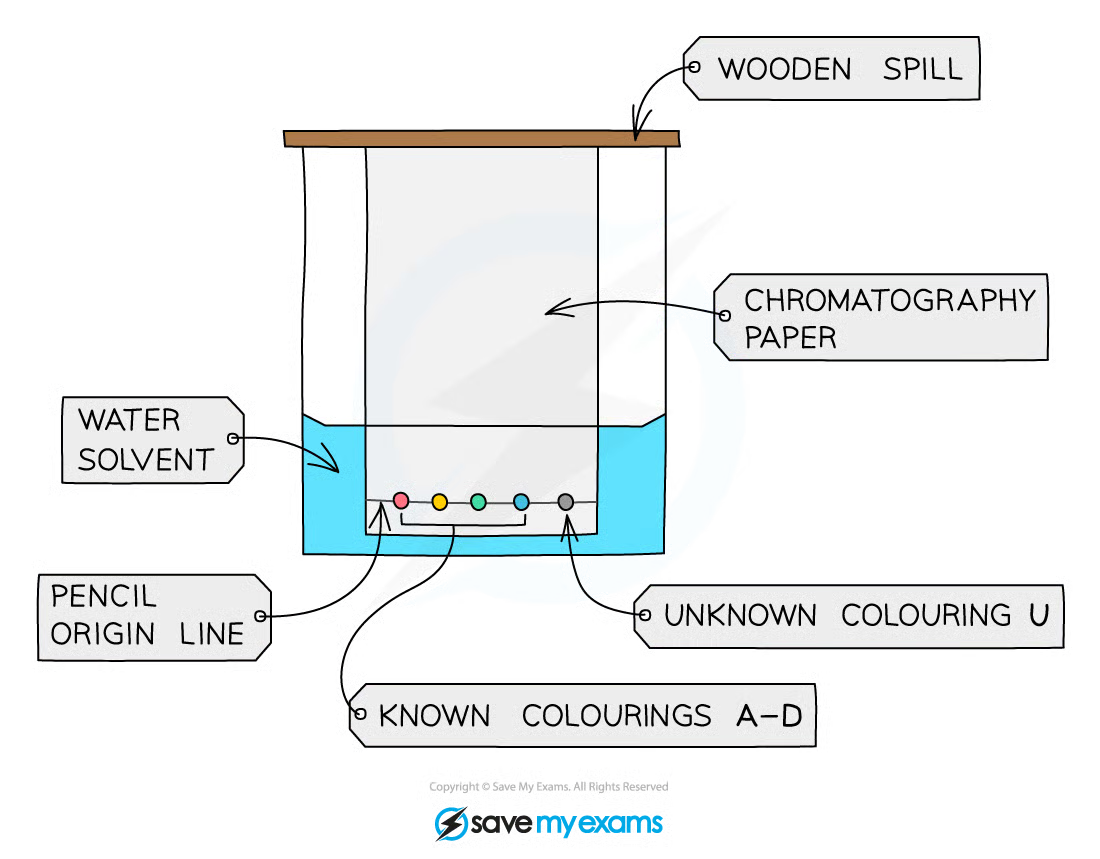
1\.13 Investigating paper chromatography using inks/food colourings
1. Use a ruler to draw a horizontal pencil line 2 cm from the end of the chromatography paper
2. Use a different capillary tube to put a tiny spot of each colouring A, B, C and D on the line
3. Use the fifth tube to put a small spot of the unknown mixture U on the line
4. Make sure each spot is no more than 2-3 mm in diameter and label each spot in pencil
5. Pour water into the beaker to a depth of no more than 1 cm and clip the top of the chromatography paper to the wooden spill. The top end is the furthest from the spots
6. Carefully rest the wooden spill on the top edge of the beaker. The bottom edge of the paper
should dip into the solvent
7. Allow the solvent to travel undisturbed at least three quarters of the way up the paper
8. Remove the paper and draw another pencil line on the dry part of the paper as close to the wet edge as possible. This is called the solvent front line
9. Measure the distance in mm between the two pencil lines. This is the distance travelled by the water solvent
10. For each of food colour A, B, C and D measure the distance in mm from the start line to the middle of the spot
29
New cards
1\.14 What is an atom?
The smallest particles of an element that consists of electrons surrounding a nucleus that contains protons and electrons
30
New cards
1\.14 What is a molecule?
A group of two or more atoms chemically bonded together forming an identifiable unit which retains the properties and composition of the substance
31
New cards
1\.15 What is the structure of an atom?
**Proton** - relative mass = 1; charge = +1
**Neutron** - relative mass = 1; charge = 0 (neutral)
**Electron** - relative mass = 1/1840; charge = -1
**Neutron** - relative mass = 1; charge = 0 (neutral)
**Electron** - relative mass = 1/1840; charge = -1
32
New cards
1\.16 What is atomic number?
The number of protons in the nucleus of an atom
33
New cards
1\.16 What is mass number?
The sum of the number of protons and neutrons in the nucleus of an atom
34
New cards
1\.16 What are isotopes?
Atoms of the same element that contain the same number of protons but a different number of neutrons, therefore, having the same atomic number but a different mass number
35
New cards
1\.16 What is relative atomic mass?
Weighted average mass of one atom of an element, taking into account the abundance of all the isotopes of that element
36
New cards
1\.17 How do you calculate the relative atomic mass of an element from isotopic abundances?
Ar = (% of isotope A x mass of isotope A + % of isotope B x mass of isotope B) ÷ 100
37
New cards
1\.18 How are elements arranged in the Periodic Table?
* In order of atomic number
* In groups (columns) and periods (rows)
* In groups (columns) and periods (rows)
38
New cards
1\.19 How do you deduce the electronic configurations of an element from its position in the Periodic Table?
* The period shows the number of electron shells
* The group shows the number of outer electrons
* The group shows the number of outer electrons
39
New cards
1\.20 How do you classify elements as metals or non-metals?
**Metals**
* 1-3 electrons in the outer shell
* Metallic bonding
* Good electrical conductors
* Basic oxides
* Many react with acids
* Malleable
* High melting point
* High boiling point
**Non-metals**
* 4-7 electrons in the outer shell
* Covalent bonding
* Poor electrical conductors
* Acidic oxides
* Do not react with acids
* Brittle
* Low melting point
* Low boiling point
* 1-3 electrons in the outer shell
* Metallic bonding
* Good electrical conductors
* Basic oxides
* Many react with acids
* Malleable
* High melting point
* High boiling point
**Non-metals**
* 4-7 electrons in the outer shell
* Covalent bonding
* Poor electrical conductors
* Acidic oxides
* Do not react with acids
* Brittle
* Low melting point
* Low boiling point
40
New cards
1\.22 How is the electronic configuration of a main group element related to its position in the Periodic Table?
Elements with the same number of electrons in the outer shell are in the same group
41
New cards
1\.23 Why do elements in the same group of the Periodic Table have similar chemical properties?
The group number of an element which is given on the periodic table indicates the number of electrons in the outer shell so they will react and bond similarly
42
New cards
1\.24 Why do the noble gases (Group 0) not readily react?
They all have full outer shells of electrons so are extremely stable
43
New cards
1\.26 How do you calculate relative formula masses from relative atomic masses?
Add up the relative atomic masses of all the atoms present in the formula
44
New cards
1\.27 What is the unit for the amount of a substance?
Mole (mol)
45
New cards
1\.28 How do you carry out calculations involving amount of substance, relative atomic mass and relative formula mass?
Amount of substance = relative atomic mass ÷ relative formula mass
46
New cards
1\.29 How do you calculate reacting masses using experimental data and chemical equations?
* Find the moles using moles = mass ÷ Mr
* Find the molar ratio
* Find the mass again
* Find the molar ratio
* Find the mass again
47
New cards
1\.30 How do you calculate percentage yield?
Percentage yield = actual yield ÷ theoretical yield x 100
48
New cards
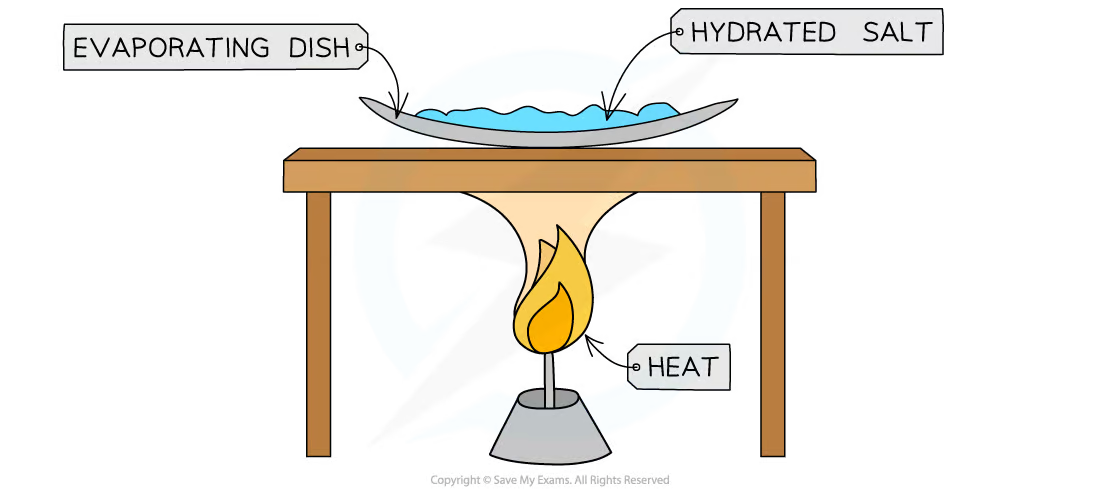
1\.31 How can the formulae of simple compounds (hydrated copper sulphate) be obtained experimentally?
* Measure the mass of evaporating dish
* Add a known mass of hydrated salt
* Heat over a Bunsen burner, gently stirring, until the blue salt turns completely white, indicating that all the water has been lost
* Record the mass of the evaporating dish and its contents
* Measure the mass of white anhydrous salt remaining
* Subtract the mass of the white anhydrous salt remaining from the mass of known hydrated salt
* Divide the mass of the copper sulphate and the water by their respective molar masses
* Simplify the ratio of water to copper sulphate
* Represent the ratio in the form ‘salt.xH2O’
* Add a known mass of hydrated salt
* Heat over a Bunsen burner, gently stirring, until the blue salt turns completely white, indicating that all the water has been lost
* Record the mass of the evaporating dish and its contents
* Measure the mass of white anhydrous salt remaining
* Subtract the mass of the white anhydrous salt remaining from the mass of known hydrated salt
* Divide the mass of the copper sulphate and the water by their respective molar masses
* Simplify the ratio of water to copper sulphate
* Represent the ratio in the form ‘salt.xH2O’
49
New cards
1\.32 What is empirical formula?
The simplest whole number ratio of the atoms of each element present in one molecule of the compound
50
New cards
1\.32 What is molecular formula?
The formula that shows the number and type of each atom in a molecule
51
New cards
1\.33 How do you calculate empirical and molecular formulae from experimental data?
**Empirical formula**
* Take the subscripts of the molecular formula and reduce them to the simplest whole number ratios
**Molecular formula**
* Calculate the relative formula mass of the empirical formula
* Divide the relative formula mass of X by the mass of the empirical formula
* Multiply each number of elements by the answer to above
* Take the subscripts of the molecular formula and reduce them to the simplest whole number ratios
**Molecular formula**
* Calculate the relative formula mass of the empirical formula
* Divide the relative formula mass of X by the mass of the empirical formula
* Multiply each number of elements by the answer to above
52
New cards
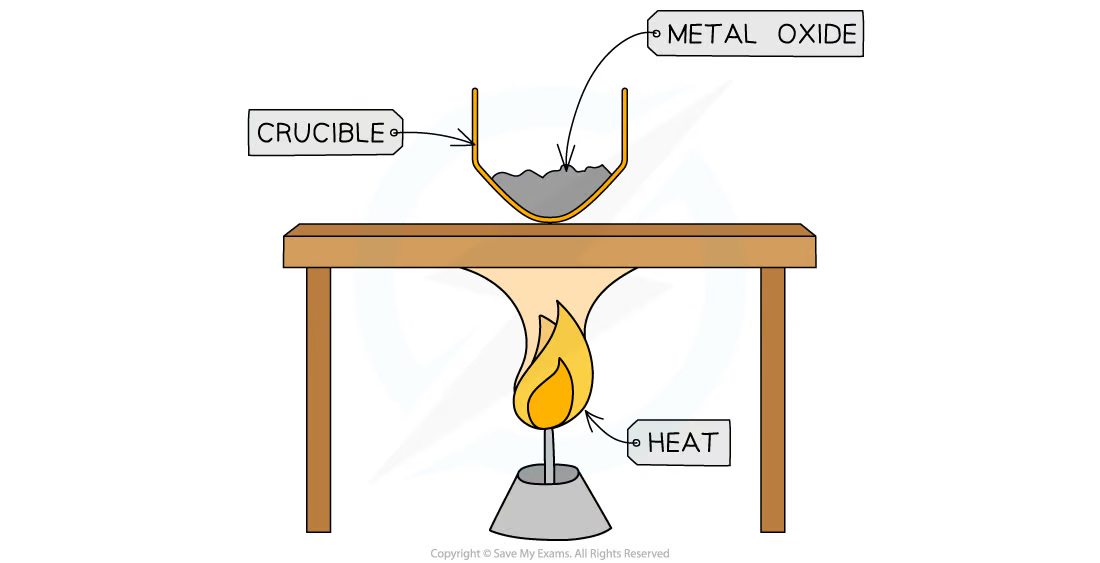
1\.36 Investigating how to determine the formula of a metal oxide by combustion
* Measure the mass of the crucible with the lid
* Add a sample of magnesium into the crucible and measure the mass with the lid (calculate the mass of the metal by subtracting the mass of the empty crucible)
* Strongly heat the crucible over a Bunsen burner for several minutes
* Lift the lid frequently to allow sufficient air into the crucible for the magnesium to fully oxidise without letting magnesium oxide smoke escape
* Continue heating until the mass of the crucible remains constant (maximum mass), indicating that the reaction is complete
* Measure the mass of the crucible and its contents (calculate the mass of metal oxide by subtracting the mass of the empty crucible)
* Subtract the mass of the crucible from magnesium and the mass of the empty crucible
* Divide each of the two masses by the relative atomic masses of the elements
* Simplify the ratio
* Represent the ratio in the form ‘MxOy‘
* Add a sample of magnesium into the crucible and measure the mass with the lid (calculate the mass of the metal by subtracting the mass of the empty crucible)
* Strongly heat the crucible over a Bunsen burner for several minutes
* Lift the lid frequently to allow sufficient air into the crucible for the magnesium to fully oxidise without letting magnesium oxide smoke escape
* Continue heating until the mass of the crucible remains constant (maximum mass), indicating that the reaction is complete
* Measure the mass of the crucible and its contents (calculate the mass of metal oxide by subtracting the mass of the empty crucible)
* Subtract the mass of the crucible from magnesium and the mass of the empty crucible
* Divide each of the two masses by the relative atomic masses of the elements
* Simplify the ratio
* Represent the ratio in the form ‘MxOy‘
53
New cards
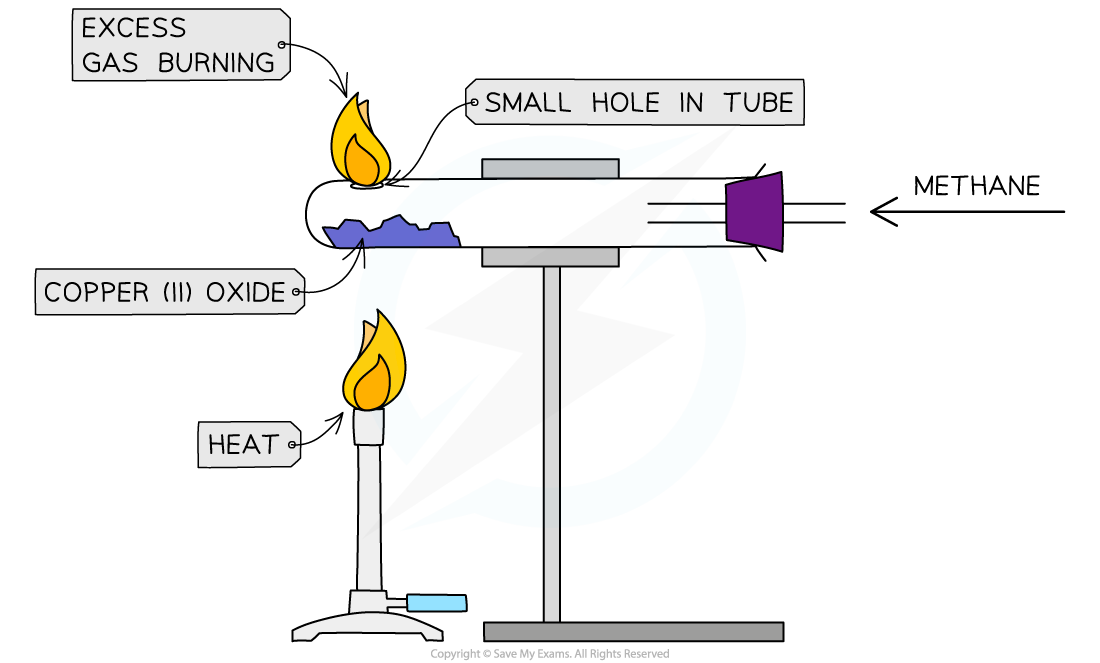
1\.36 Investigating how to determine the formula of a metal oxide by reduction
* Measure mass of the empty boiling tube
* Place metal oxide into a horizontal boiling tube and measure the mass again
* Support the tube in a horizontal position held by a clamp
* A steady stream of natural gas(methane) is passed over the copper(II)oxide and the excess gas is burned off
* The copper(II)oxide is heated strongly using a Bunsen burner
* Heat until metal oxide completely changes colour, meaning that all the oxygen has been removed
* Measure mass of the tube remaining metal powder and subtract the mass of the tube
* Measure mass of the remaining metal powder
* Subtract mass of the remaining metal powder from the mass of metal oxide
* Divide each of the two masses by the relative atomic masses of elements
* Simplify the ratio
* Represent the ratio into the form ‘MxOy‘
* Place metal oxide into a horizontal boiling tube and measure the mass again
* Support the tube in a horizontal position held by a clamp
* A steady stream of natural gas(methane) is passed over the copper(II)oxide and the excess gas is burned off
* The copper(II)oxide is heated strongly using a Bunsen burner
* Heat until metal oxide completely changes colour, meaning that all the oxygen has been removed
* Measure mass of the tube remaining metal powder and subtract the mass of the tube
* Measure mass of the remaining metal powder
* Subtract mass of the remaining metal powder from the mass of metal oxide
* Divide each of the two masses by the relative atomic masses of elements
* Simplify the ratio
* Represent the ratio into the form ‘MxOy‘
54
New cards
1\.37 How are ions formed?
When atoms lose or gain electrons
55
New cards
1\.38 What is the charge of silver?
\+
56
New cards
1\.38 What is the charge of copper?
2+
57
New cards
1\.38 What is the charge of lead?
2+
58
New cards
1\.38 What is the charge of zinc?
2+
59
New cards
1\.38 What is the charge of hydrogen?
\+
60
New cards
1\.38 What is the charge of hydroxide?
\-
61
New cards
1\.38 What is the charge of ammonium?
\+
62
New cards
1\.38 What is the charge of carbonate?
2-
63
New cards
1\.38 What is the charge of nitrate?
\-
64
New cards
1\.38 What is the charge of sulphate?
2-
65
New cards
1\.41 What is ionic bonding in terms of electrostatic attractions?
An electrostatic force of attraction between oppositely charged ions
66
New cards
1\.42 Why do compounds with giant ionic lattices have high melting and boiling points?
Strong electrostatic forces of attraction between oppositely charged ions require lots of energy to overcome
67
New cards
1\.43 When do ionic compounds conduct electricity?
When molten or in aqueous solution
68
New cards
1\.44 How is a covalent bond formed between atoms?
By the sharing of a pair of electrons
69
New cards
1\.45 What is a covalent bond in terms of electrostatic attractions?
An electrostatic force of attraction between two nuclei and a shared pair of electrons
70
New cards
1\.47 Why are substances with a simple molecular structures gases or liquids, or solids with low melting and boiling points?
Weak forces of attraction between molecules require little energy to overcome
71
New cards
1\.48 Why does the melting and boiling points of substances with simple molecular structures increase, in general, with increasing relative molecular mass?
Because larger molecules have more forces of attraction between them which, although weak, must be overcome if the substance is to boil, and larger molecules have more attractions which must be overcome
72
New cards
1\.49 Why are substances with giant covalent structures solids with high melting and boiling points?
Because strong covalent bonds between atoms require lots of energy to break
73
New cards
1\.50 How does the structure of diamond influence its physical properties?
* Allotrope of carbon
* Each carbon atom bonds with four other carbons, forming a tetrahedron
* Does not conduct electricity because all the outer shell electrons in carbon are held in the four covalent bonds around each carbon atom
* High melting point because of strong covalent bonds
* Extremely hard because of very, strong identical covalent bonds and no intermolecular forces
* Each carbon atom bonds with four other carbons, forming a tetrahedron
* Does not conduct electricity because all the outer shell electrons in carbon are held in the four covalent bonds around each carbon atom
* High melting point because of strong covalent bonds
* Extremely hard because of very, strong identical covalent bonds and no intermolecular forces
74
New cards
1\.50 How does the structure of graphite influence its physical properties?
* Allotrope of carbon
* Each carbon atom in graphite is bonded to three others forming layers of hexagons, leaving one free electron per carbon atom
* Conducts electricity and heat because of free electrons migrate along the layers and are free to move and carry charge
* High melting point because of strong covalent bonds
* Soft and slippery because of weak intermolecular forces of attraction
* Each carbon atom in graphite is bonded to three others forming layers of hexagons, leaving one free electron per carbon atom
* Conducts electricity and heat because of free electrons migrate along the layers and are free to move and carry charge
* High melting point because of strong covalent bonds
* Soft and slippery because of weak intermolecular forces of attraction
75
New cards
1\.50 How does the structure of C60 fullerene influence its physical properties?
* Part of a group of carbon allotropes which consist of molecules that form hollow tubes or spheres
* Can be used to trap other molecules by forming around the target molecule and capturing it, making them useful for targeted drug delivery systems
* They also have a huge surface area and are useful for trapping catalyst molecules onto their surfaces making them easily accessible to reactants so catalysis can take place
* Some fullerenes are excellent lubricants and are starting to be used in many industrial processes
* The first fullerene to be discovered was buckminsterfullerene which is affectionately referred to as a “buckyball”
* In this fullerene, 60 carbon atoms are joined together forming 20 hexagons and 12 pentagons which produce a hollow sphere that is the exact shape of a soccer ball
* Can be used to trap other molecules by forming around the target molecule and capturing it, making them useful for targeted drug delivery systems
* They also have a huge surface area and are useful for trapping catalyst molecules onto their surfaces making them easily accessible to reactants so catalysis can take place
* Some fullerenes are excellent lubricants and are starting to be used in many industrial processes
* The first fullerene to be discovered was buckminsterfullerene which is affectionately referred to as a “buckyball”
* In this fullerene, 60 carbon atoms are joined together forming 20 hexagons and 12 pentagons which produce a hollow sphere that is the exact shape of a soccer ball
76
New cards
1\.51 Do covalent compounds do usually conduct electricity?
No
77
New cards
2.1 How do the similarities in the reactions of Group 1 elements with water provide evidence for their recognition as a family of elements?
**Lithium** - effervescence, floats, moves across the water, shrinks and disappears
**Sodium** - effervescence, floats, moves across the water, shrinks and melts into a shiny ball
**Potassium** - effervescence, floats, moves across the water, shrinks, melts into a shiny ball and ignites with a lilac flame
**Sodium** - effervescence, floats, moves across the water, shrinks and melts into a shiny ball
**Potassium** - effervescence, floats, moves across the water, shrinks, melts into a shiny ball and ignites with a lilac flame
78
New cards
2.2 How do the differences between the reactions of these elements with air and water provide evidence for the trend in reactivity in Group 1?
**Reaction with oxygen** - all form a white solid, flame colour gets cooler (therefore flame is hotter) down the group
**Reaction with water** - reaction becomes more intense down the group
Reactivity increases down the group because the reactions become more vigorous
**Reaction with water** - reaction becomes more intense down the group
Reactivity increases down the group because the reactions become more vigorous
79
New cards
2.3 Using the knowledge of trends in Group 1, can you predict the properties of other alkali metals?
Reaction of alkali metals with air and water becomes more vigorous as you go down the group
80
New cards
2.5 What are the colours, physical states (at room temperature) and trends in physical properties of Group 7 elements?
**Fluorine** - pale yellow gas
**Chlorine** - pale green gas
**Bromine** - orange liquid
**Iodine** - grey solid (purple vapour)
**Astatine** - black solid
Melting and boiling points increase down the group
**Chlorine** - pale green gas
**Bromine** - orange liquid
**Iodine** - grey solid (purple vapour)
**Astatine** - black solid
Melting and boiling points increase down the group
81
New cards
2.6 Using the knowledge of trends in Group 7, can you predict the properties of other halogens?
* The melting and boiling point of halogens increases as you go down the group
* The state of halogens becomes harder and more set as you go down the group
* The colour of halogens becomes darker as you go down the group
* The state of halogens becomes harder and more set as you go down the group
* The colour of halogens becomes darker as you go down the group
82
New cards
2.7 How do displacement reactions involving halogens and halides provide evidence for the trend in reactivity in Group 7?
The elements higher up in the group will displace the elements below them from aqueous solutions
83
New cards
2.9 What are the approximate percentages by volume of the four most abundant gases in dry air?
**Nitrogen** - 78%
**Oxygen** - 21%
**Argon** - 0.9%
**Carbon dioxide** - 0.04%
**Oxygen** - 21%
**Argon** - 0.9%
**Carbon dioxide** - 0.04%
84
New cards
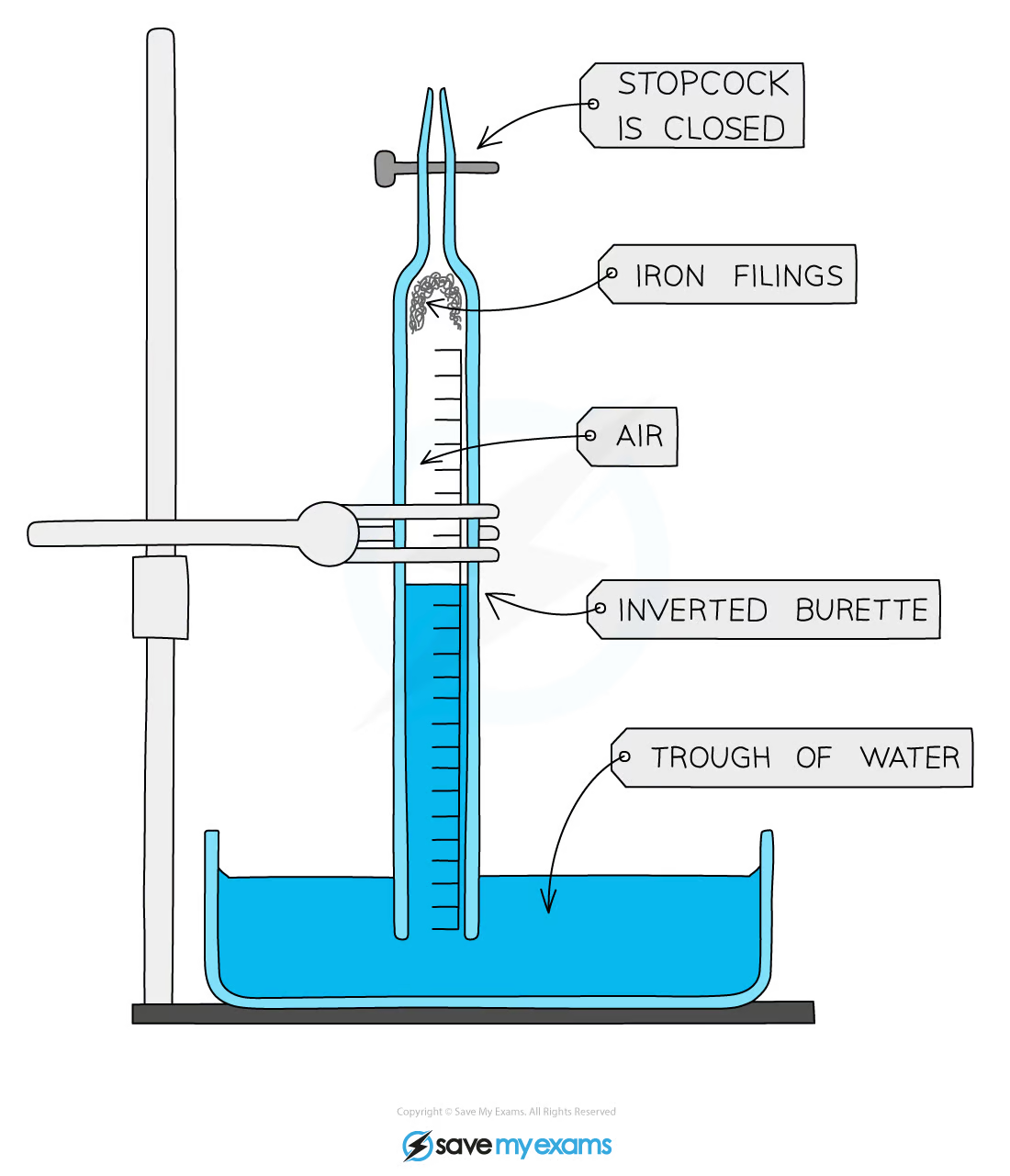
2.10 How do you determine the percentage by volume of oxygen in the air using the reactions of metals (e.g. iron) with air?
* Place wet iron filings at the end of a burette
* Use a clamp to hold the burette vertically in the trough of water
* Measure and note the starting height of the water level in the burette
* Leave apparatus for several days
* Measure and note the final height of the water level in the burette
As the water level will rise to replace the volume of oxygen lost during the reaction to form iron (II) oxide, a constant water level will be reached as the iron filings will fully oxidise with oxygen in the air
* Use a clamp to hold the burette vertically in the trough of water
* Measure and note the starting height of the water level in the burette
* Leave apparatus for several days
* Measure and note the final height of the water level in the burette
As the water level will rise to replace the volume of oxygen lost during the reaction to form iron (II) oxide, a constant water level will be reached as the iron filings will fully oxidise with oxygen in the air
85
New cards
2.10 How do you determine the percentage by volume of oxygen in the air using the reactions of non-metals (e.g. phosphorus) with air?
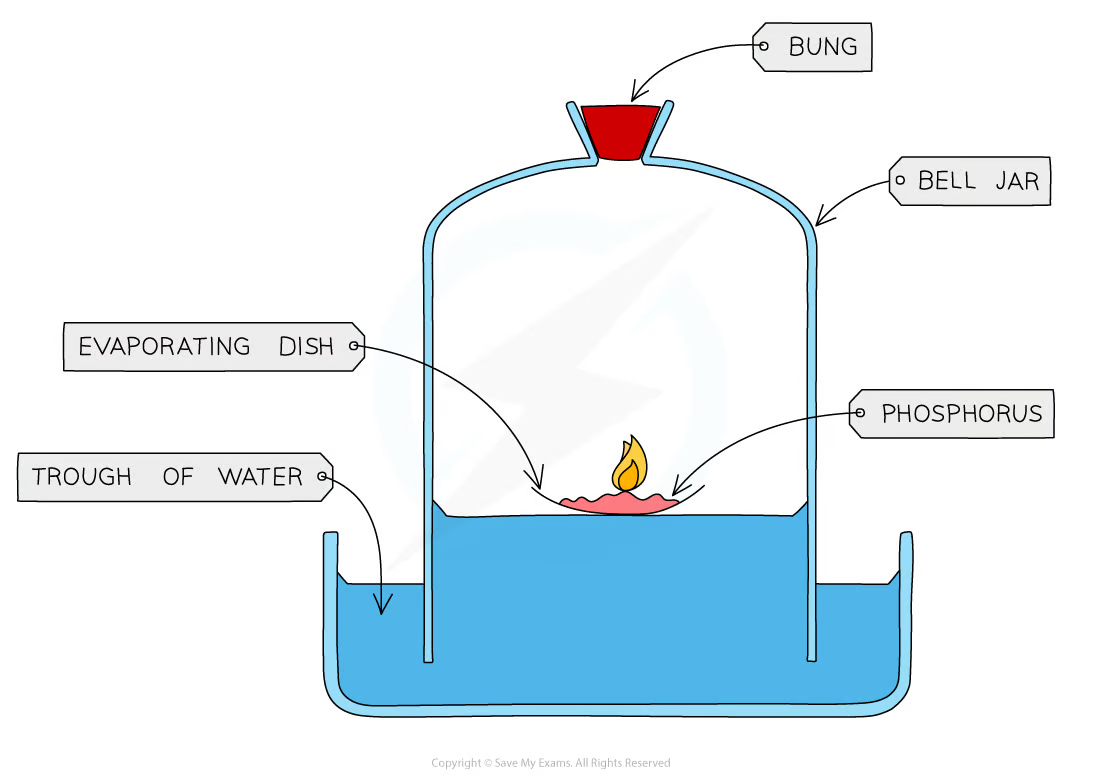
* Add phosphorus into an evaporating dish and place it on a trough of water
* Ignite phosphorus using a candle
* Cover evaporating dish with a bell jar
* Measure and note the starting height of the water level in the bell jar
* Leave apparatus for several days
* Measure and note the final height of the water level in the bell jar
As the water level will rise to replace the volume of oxygen lost during this reaction, a constant level will be reached as phosphorus will use up all the oxygen in the air to burn
* Ignite phosphorus using a candle
* Cover evaporating dish with a bell jar
* Measure and note the starting height of the water level in the bell jar
* Leave apparatus for several days
* Measure and note the final height of the water level in the bell jar
As the water level will rise to replace the volume of oxygen lost during this reaction, a constant level will be reached as phosphorus will use up all the oxygen in the air to burn
86
New cards
2.11 How do you describe the combustion of magnesium, hydrogen and sulphur in oxygen?
**Magnesium** - intense white light, white powder produced
**Hydrogen** - exothermic, water is produced
**Sulphur** - blue flame, colourless gas produced
**Hydrogen** - exothermic, water is produced
**Sulphur** - blue flame, colourless gas produced
87
New cards
2.12 How do you describe the formation of carbon dioxide from the thermal decomposition of metal carbonates, including copper (II) carbonate?
When copper (II) carbonate is thermally decomposed, it turns from green (copper (II) carbonate) to black (copper oxide) as carbon dioxide is lost
88
New cards
2.13 What is carbon dioxide?
A greenhouse gas that increasing amounts of in the atmosphere may contribute to climate change
89
New cards
2.14 Determining the approximate percentage by volume of oxygen in air using a metal or a non-metal
**Metal**
* Place wet iron filings at the end of a burette
* Use a clamp to hold the burette vertically in the trough of water
* Measure and note the starting height of the water level in the burette
* Leave apparatus for several days
* Measure and note the final height of the water level in the burette
As the water level will rise to replace the volume of oxygen lost during the reaction to form iron (II) oxide, a constant water level will be reached as the iron filings will fully oxidise with oxygen in the air
**Non-metal**
* Add phosphorus into an evaporating dish and place it on a trough of water
* Ignite phosphorus using a candle
* Cover evaporating dish with a bell jar
* Measure and note the starting height of the water level in the bell jar
* Leave apparatus for several days
* Measure and note the final height of the water level in the bell jar
As the water level will rise to replace the volume of oxygen lost during this reaction, a constant level will be reached as phosphorus will use up all the oxygen in the air to burn
**The percentage volume of oxygen** = (volume of oxygen reacted ÷ initial volume of air) × 100
* Place wet iron filings at the end of a burette
* Use a clamp to hold the burette vertically in the trough of water
* Measure and note the starting height of the water level in the burette
* Leave apparatus for several days
* Measure and note the final height of the water level in the burette
As the water level will rise to replace the volume of oxygen lost during the reaction to form iron (II) oxide, a constant water level will be reached as the iron filings will fully oxidise with oxygen in the air
**Non-metal**
* Add phosphorus into an evaporating dish and place it on a trough of water
* Ignite phosphorus using a candle
* Cover evaporating dish with a bell jar
* Measure and note the starting height of the water level in the bell jar
* Leave apparatus for several days
* Measure and note the final height of the water level in the bell jar
As the water level will rise to replace the volume of oxygen lost during this reaction, a constant level will be reached as phosphorus will use up all the oxygen in the air to burn
**The percentage volume of oxygen** = (volume of oxygen reacted ÷ initial volume of air) × 100
90
New cards
2.15 How can metals be arranged in a reactivity series based on their reactions with water and dilute hydrochloric or sulphuric acid?
Metals at the top of the reactivity series will react more vigorously with water and dilute acids as they are more reactive, whilst metals at the bottom of the reactivity series will react slowly or will not react with water and dilute acids as they are less reactive
Elements below **calcium** in the reactivity series will not react with **water** and elements below **iron** in the reactivity series will not react with **dilute acids**
Elements below **calcium** in the reactivity series will not react with **water** and elements below **iron** in the reactivity series will not react with **dilute acids**
91
New cards
2.16 How can metals be arranged in a reactivity series based on their displacement reactions between metals and metal oxides, and metals and aqueous solutions of metal salts?
Reactivity of metals increases as you go up the reactivity series, therefore a metal will only displace another metal that is below it in the reactivity series
92
New cards
2.17 What is the reactivity series?
**P**lease (Potassium)
**S**end (Sodium)
**L**ions, (Lithium)
**C**ats, (Calcium)
**M**onkeys, (Magnesium)
**A**nd (Aluminium)
**C**ute (Carbon)
**Z**ebras (Zinc)
**I**nto (Iron)
**H**ot (Hydrogen)
**C**ountries. (Copper)
**S**igned, (Silver)
**G**ordon (Gold)
**S**end (Sodium)
**L**ions, (Lithium)
**C**ats, (Calcium)
**M**onkeys, (Magnesium)
**A**nd (Aluminium)
**C**ute (Carbon)
**Z**ebras (Zinc)
**I**nto (Iron)
**H**ot (Hydrogen)
**C**ountries. (Copper)
**S**igned, (Silver)
**G**ordon (Gold)
93
New cards
2.18 What are the conditions under which iron rusts?
In the presence of water and oxygen
94
New cards
2.19 How may the rusting of iron be prevented?
**Barrier methods** - rust can be prevented by coating iron with barriers that prevent it from coming into contact with water and oxygen e.g. grease, oil, paint and plastic
**Galvanising** - coating the iron with a layer of zinc
**Sacrificial protection** - using elements higher than iron in the reactivity series
**Galvanising** - coating the iron with a layer of zinc
**Sacrificial protection** - using elements higher than iron in the reactivity series
95
New cards
2.20 What does oxidation mean?
Loss of electrons and gain of oxygen
96
New cards
2.20 What does reduction mean?
Gain of electrons and loss of oxygen
97
New cards
2.20 What does being an oxidising agent mean?
Substance that gains electrons and loses oxygen
98
New cards
2.20 What does being a reducing agent mean?
Substance that loses electrons and gains oxygen
99
New cards
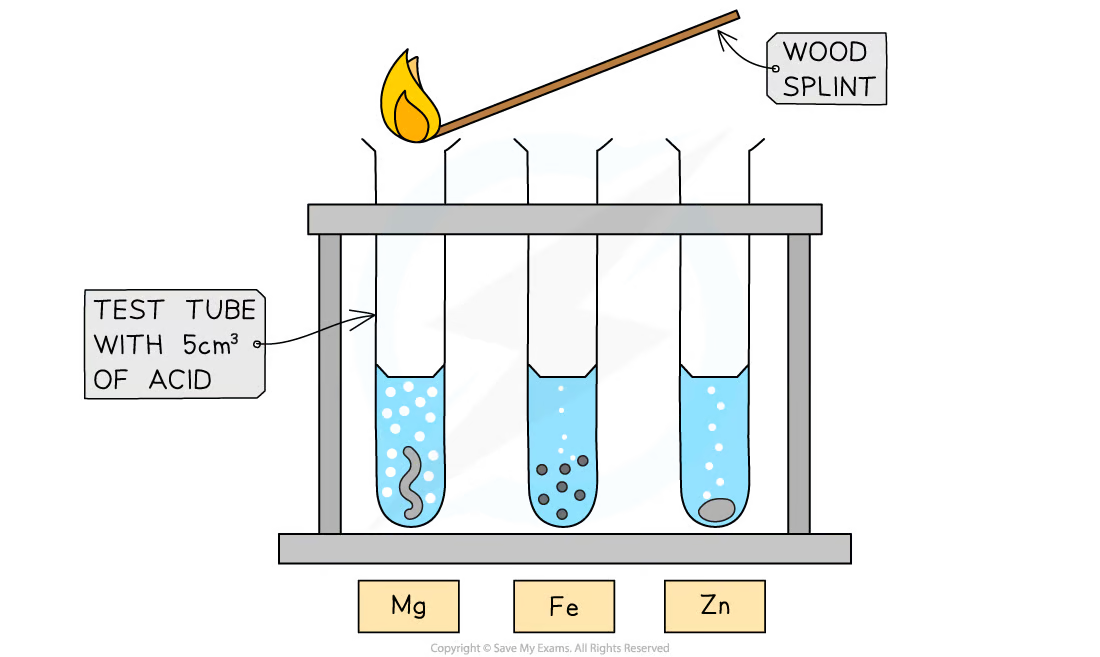
2.21 Investigating reactions between dilute hydrochloric and sulphuric acids and metals (e.g. magnesium, zinc and iron)
**M**etal **+ A**cid → **S**alt + **H**ydrogen
* Add acid to a test tube
* Add metal into the test tube
* Observe the reaction and the rate at which bubbles are produced
* Add acid to a test tube
* Add metal into the test tube
* Observe the reaction and the rate at which bubbles are produced
100
New cards
2.28 How do you describe the use of different indicators to distinguish between acidic and alkaline solutions?
**Red litmus** - stays red in acids and neutral solutions, turns blue in alkalis
**Blue litmus** - turns red in acids, stays blue in neutral solutions and alkalis
**Phenolphthalein** - colourless in acids and neutral solutions, turns pink in alkalis
**Methyl orange** - red in acids, yellow in neutral solutions and alkalis
**Blue litmus** - turns red in acids, stays blue in neutral solutions and alkalis
**Phenolphthalein** - colourless in acids and neutral solutions, turns pink in alkalis
**Methyl orange** - red in acids, yellow in neutral solutions and alkalis