ex 8 methyl salicylate
1/63
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
64 Terms
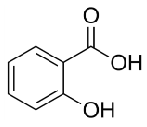
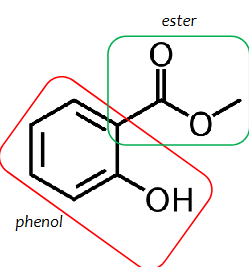
methyl salicylate
also known as wintergreen oil or betula oil
methyl salicylate
an ester volatile oil that has a characteristic flavor and odor (minty)
salicylic acid
precursor of methyl salicylate
maceration and subsequent steam distillation
methyl salicylate can be produced naturally by ___
wintergreen leaves
Gaultheria procumbens
SWEET BIRCH TREE BARK
Betula lenta
MEADOWSWEET LEAVES
Filipendula ulmaria
Auguste André Thomas Cahours
methyl salicylate was first isolated from the plant Gaultheria procumbens L. in 1843 by the French chemist ___
methyl salicylate
an ester of salicylic acid and methanol
• rubefacient in deep heating liniments to treat joint and muscular pain
• counter-irritant
• analgesic
use of methyl salicylate in high concentration
• flavoring agent in chewing gum and mints (0.04% and below)
• provides fragrance in other products
• odor-masking in some pesticides
use of methyl salicylate in low concentration
liniment
(methyl salicylate) efficascent oil
ointment
(methyl salicylate) katinko
liniment
(methyl salicylate)omega pain killer
cream
(methyl salicylate) ben-gay pain relieving
patch
(methyl salicylate) salonpas pain relief
embrocation
(methyl salicylate) white flower
7; 23
a single teaspoon (5 mL) of methyl salicylate contains _ g of salicylate, which is equivalent to more than __ 300 mg aspirin tablets
N&V, hyperventilation and tinnitus
salicylism
-
methyl salicylate can also cause skin irritation and skin rashes
salicylic acid
limiting reactant for syntheis of methyl salicylate
salicylic acid
reacts with methanol in the presence of sulfuric acid to form methyl salicylate
methanol
salicylic acid reacts with __ in the presence of sulfuric acid to form methyl salicylate
ester group
salicylic acid reacts with methanol in the presence of sulfuric acid to form methyl salicylate
the carboxylic acid group is replaced with __ upon reaction
salicylic acid
15.2 g
ilang grams ung ginamit na salicylic acid
36ml
ilang ml ung ginamit na methanol
water
• the methyl group of the methanol attaches to carboxylate ion forming an ester
• the hydroxyl group forms bond with H+ producing __
3.5ml
ilang ml ung ginamit na concentrated sulfuric acid
conc. sulfuric acid
used as catalyst to accelerate the reaction of salicylic acid with methanol
Petroluem Ether
used to extract the methyl salicylate
organic solvent(like petroleum ether)
• methyl salicylate is more soluble in __; other components in the mixture especially if very polar, will be drawn into water
• the aqueous layer is drained, leaving the organic solvent where the methyl salicylate is dissolved
• removed from the mixture by steam bath
steam bath
petroleum ether is removed from the mixture by __
20% sodium carbonate
used to remove the unreacted salicylic acid
20% sodium carbonate
it reacts with salicylic acid to form sodium salicylate with carbon dioxide as a by-product
carbon dioxide
sodium carbonate reacts with salicylic acid to form sodium salicylate with as __ a by-product
-
• sodium carbonate is used to remove the unreacted salicylic acid
• it reacts with salicylic acid to form sodium salicylate with carbon dioxide as a by-product
• the ionic sodium salicylate would drawn into the aqueous layer (soluble) and can be removed from the extraction mixture
anhydrous magnesium sulfate
used to remove the remaining water in the mixture of the organic solvent (petroleum ether) and the desired product (methyl salicylate)
anhydrous magnesium sulfate
it acts as a drying agent, dehydrating the mixture leaving the organic layer free from water and other water-soluble impurities
salicylic acid, methanol, sulfuric acid
synthesis of methyl salicylate
• __ are mixed all together in an Erlenmeyer flask
• boiling chips are added to prevent bumping of solution
• solution is refluxed for 1 hour
1 hour
synthesis of methyl salicylate
• salicylic acid, methanol, sulfuric acid are mixed all together in an Erlenmeyer flask
• boiling chips are added to prevent bumping of solution
• solution is refluxed for __
deprotonated
Fischer Esterification
• the carboxylic group of the salicylic acid is __ and the methyl group from the methanol reacts to form an ester, which is the methyl salicylate
• reaction is catalyzed by sulfuric acid
Fischer Esterification
reaction in synthesis of methyl salicylate
ice bath; petroleum ether; distilled water; 20% sodium carbonate
extraction in synthesis of methyl salicylate
• after heating, solution is cooled in an __
• __ is used to extract the methyl salicylate
• __ is added to wash off polar compounds
• __ is added to remove excess unreacted salicylic acid
anhydrous magnesium sulfate
drying & filtration in synthesis of methyl salicylate
• extract is dried using __
• it is then filtered in a pre-weighed evaporating dish
• solvent is removed using steam bath
• methyl salicylate is weighed, and percentage yield is computed
ester + water
salicylic acid + methanol =
anhydrous magnesium sulfate
synthesis of methyl salicylate
Removing water formed by adding a drying agent like __ to the reaction mixture. This shifts the equilibrium to the right.
• color: clear colorless or slightly yellowish
• odor: minty (wintergreen odor)
• appearance: oily, slightly viscous liquid
physical test of methyl salicylate (color, odor, appearance)
hydroxamic acid
ester + hydroxylamine (1ml) =
maroon
esters when heated with hydroxylamine produce hydroxamic acids that reacts with ferric ion to form intensely colored complexes, commonly dark __ in color
ferric hydroxamate test
related to the phenol test wherein compounds with high enolic character gives colored complexes with ferric ion
no color/ clear faint yellow
a preliminary test must be performed to check if compound produces enough enol to form colored complexes with ferric ion (continue if __ color only)
__
esters do not form colored complex with ferric ion in the preliminary test
oil of wintergreen, betula oil, gaultheria oil
methyl salicylate synomyms
Methyl-2-hydroxybenzoate
IUPAC name of methyl salicylate
C8H8C3
molecular formula of methyl salicylate
152.15 g/mol
molecular weight of methyl salicylate
222C
boiling point of methyl salicylate
-8.6C
melting point of methyl salicylate
methyl salicylate
0.07 g per 100 mL water at 20°C
solubility of methyl salicylate
1.184g/cm3
density of methyl salicylate
sensitive to light and heat
stability of methyl sallicylate
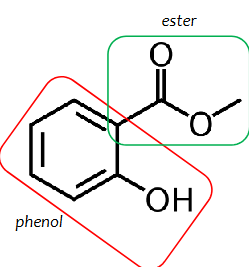
phenol with an attached ester group on ortho position
structure of methyl salicylate