2.1 - Introduction to Chemistry
Lesson 2.1.1 - Matter
Matter - Anything that takes up space and has mass
Element - A substance that cannot be broken down into other substances by chemical reaction
Compound - A substance consisting of 2 or more elements in a fixed ratio
Carbon, Oxygen, Nitrogen, and Hydrogen make up 98% of all living matter.
Atom - Basic unit of an element
Atomic Number - The number of protons in an atom of an element
Atomic nuclei contain protons and neutrons
Number of protons + neutrons = atomic mass
Electrons determine how an atom interacts with other atoms
- Exist in specific energy levels around the nucleus called electron shells
- Each electron shell holds a specific number of electrons
- Number of electrons in the outer shell determines the chemical properties of an atom
- Atoms are chemically reactive when their valence shells are not filled to the max number of electrons
Isotopes - Same atomic number but different mass numbers (e.g. Carbon-12, Carbon-13, Carbon-14, etc.)
- Uses of isotopes:
- Carbon-14 is used for radioactive dating (monitor decay)
- Radioactive decay measurements involve the use of half-life (the time for half the amount of an isotope to decay)
- Half-life is constant, it cannot be altered by changes in pressure or temperature and depends solely on the nature of the radioactive nucleus.
- In biological research as tracers (e.g. Phosphorus-32, Sulfur-35, Nitrogen-15)
- Short half-lives
- Iodine-123 can be used to study the thyroid gland, such as its shape. A cancerous thyroid has a very different shape to a normal thyroid gland and this iodine tracer shows any change in shape.
Lesson 2.1.2 - Chemical Bonds and Chemical Reactions
Atoms of reactive elements tend to combine into compounds and molecules by forming chemical bonds
The 4 most important chemical interactions in biological molecules are: Ionic bonds, covalent bonds, hydrogen bonds, dipole forces
Chemical reactions occur when atoms or molecules interact to form new bonds or break old ones.
Intra-molecular Interactions - Bonding of atoms WITHIN a molecule
Inter-molecular Interactions - Attractions BETWEEN molecules
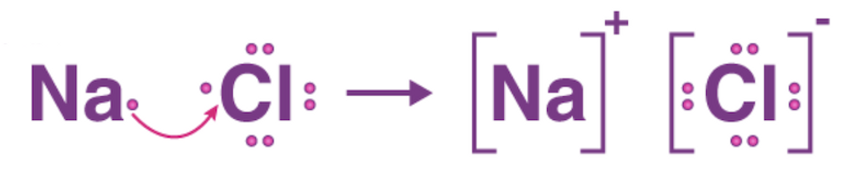
Ionic Bonding
Involve the transfer of electrons (e.g. Na + Cl = NaCl)
Electron transfer results in two ions of opposite charges
Covalent Bonding
Involve the sharing of electrons
Occurs when atoms do not have a strong tendency to transfer electrons
Atoms fill their outer shells by sharing electrons between them
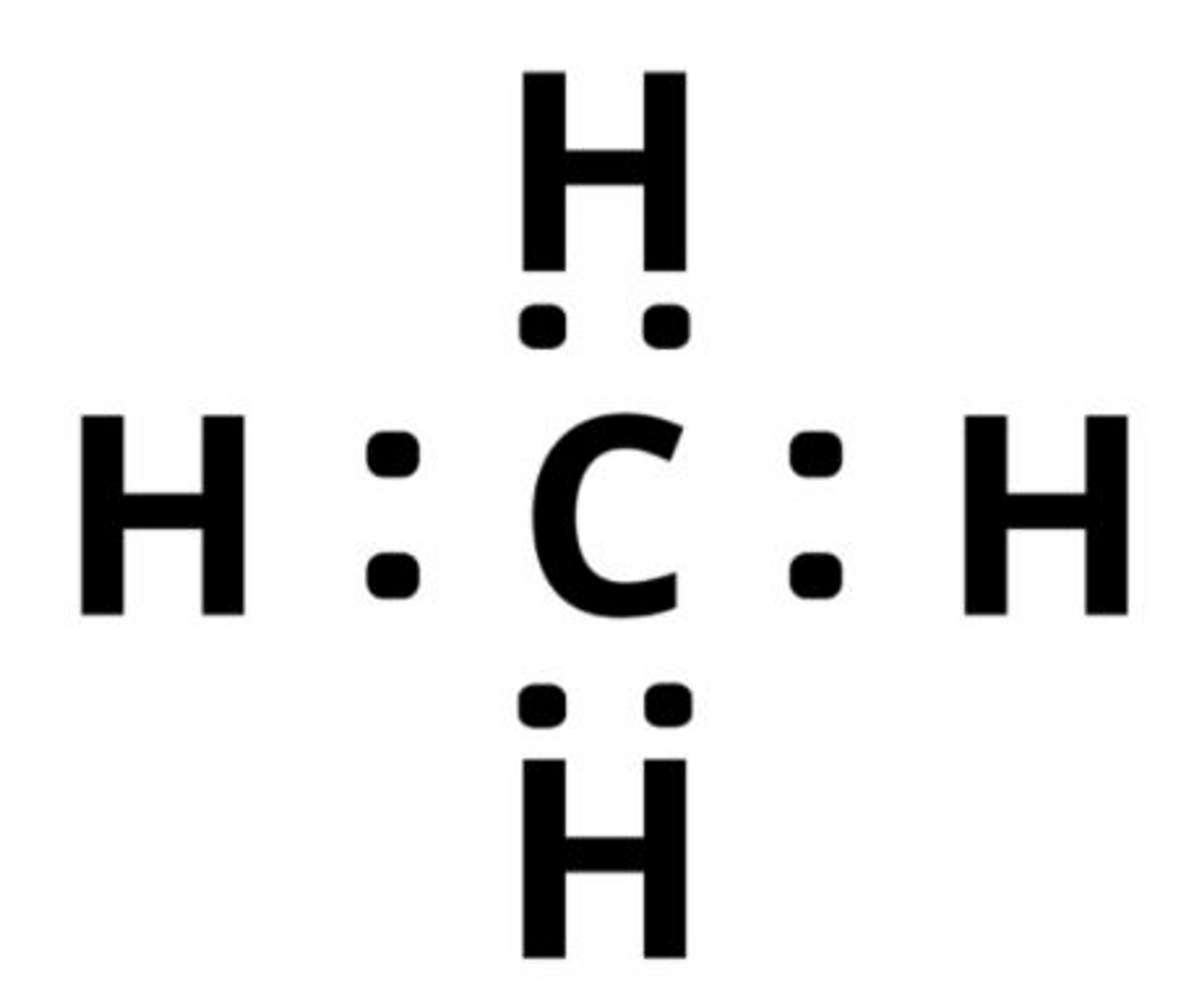
The number of covalent bonds an atom can form equals the number of electrons needed to fill the outer shell.
Asymmetrical Electron Sharing
Unequal sharing of electrons along a covalent bond results in an asymmetrical charge in a neutral molecule.
- Known as a dipole
Intermolecular interactions include dipole forces, hydrogen bonds, and Van der Waals forces
Dipole forces - Attraction between oppositely charged dipoles brings molecules closer together
The strength of dipole forces affects molecular properties such as boiling point
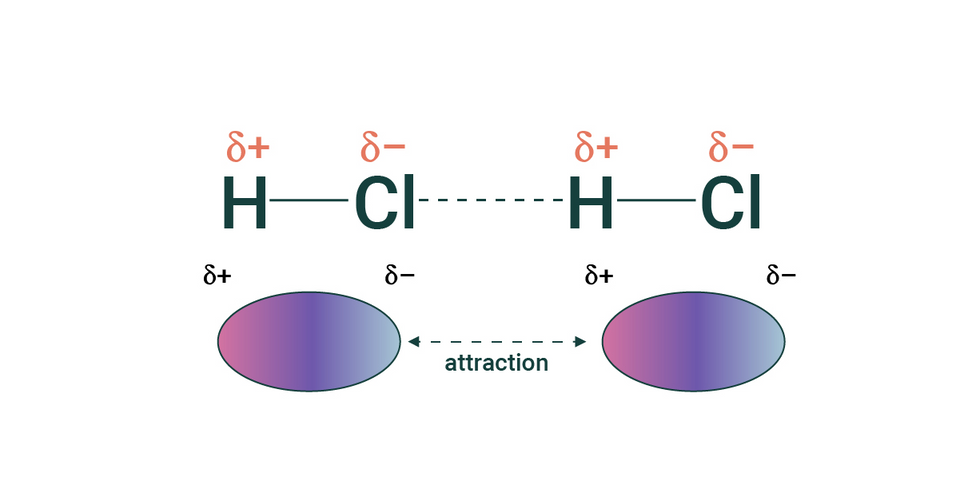
Hydrogen Bonding - The most significant dipolar attraction in biology (more about it in 2.1.3)
Lesson 2.1.3 - Water: The Molecule that Supports All Life
Water is a biological solvent
Living organisms need water more than any other substance
Most cells are surrounded by and composed of 70-95% water
Water is a polar molecule - having opposite charges on each end
Polarity allows water molecules to form hydrogen bonds with each other
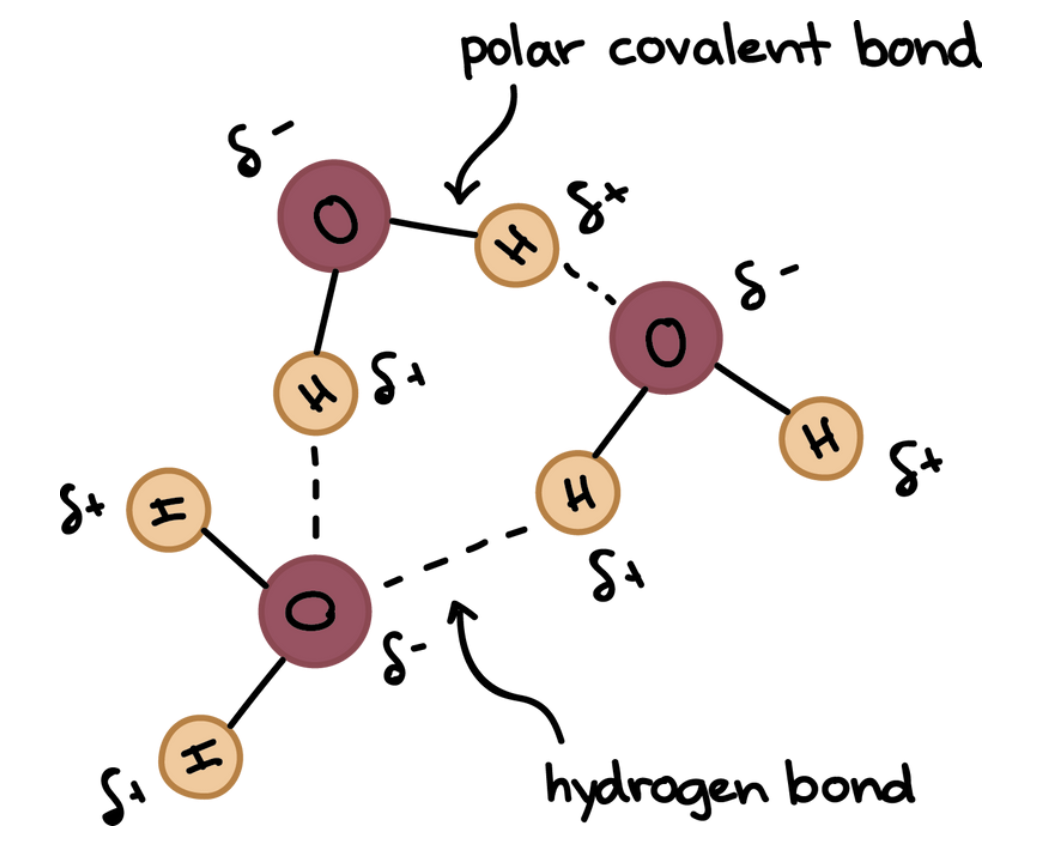
Hydrogen can form hydrogen bonds with hydrogen, oxygen, nitrogen, and fluorine atoms
Hydrogen bonds between water molecules form a water lattice affecting properties such as density, heat absorption, cohesion, and surface tension.
Polarity of water molecules contributes to formation of distinct polar and non-polar environments critical for cell organization.
- Polar environment → Hydrogen bonding does occur
- Non-polar Environment → Hydrogen bonding does not occur
Water is a solvent for charged or polar molecules
Water molecules can separate into hydrogen and hydroxyl ions
Ice floats because the molecules get arranged into a lattice structure with hydrogen bonds when it freezes, causing it to be less dense than liquid water.
Cohesion - Water molecules “stick” to each other, due to the hydrogen bond lattice, causing surface tension
Adhesion - Water molecules “stick” to other surfaces, by forming hydrogen bonds with charged and polar groups
Capillary Action - Cohesion and adhesion give water the ability to flow against gravity
Water has a strong resistance to temperature change due to hydrogen bonding
- Water can store a large amount of heat with a small increase in temperature
- Evaporation prevents overheating
Water molecules are small and strongly polar, coating the surfaces of polar molecules to form a hydration layer.
- Hydration layers reduce attractions between molecules or ions and promote their entry into solution.
- Water (solvent) surrounds the dissolved substance (solute) preventing the polar molecules from re-associating
Acids and Bases
In aqueous systems, most H2O molecules are intact, but some molecules break apart into H+ and OH- ions
Acids release H+ ions when dissolved in water, increasing H+ concentration
Bases (or alkalis) are H+ acceptors, reducing H+ concentrations in a solution.
Many bases release OH- ions that can combine with H+ to form H2O molecules
Acidity (the H+ concentration) of a solution is measured using the pH scale
pH ranges from 0 (most acidic) to 14 (most basic)
pH scale is a logarithmic scale (by x10)
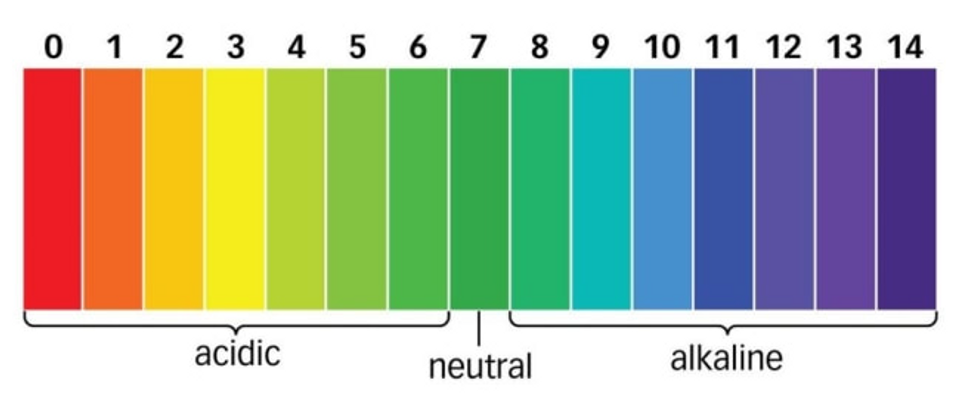
Even a slight change in normal pH can be harmful to organic life.
- Organisms maintain their internal pH through buffering
- Biological fluids contain buffers that prevent drastic changes in pH by regulating H+ concentrations
- Environmental changes in pH can have a profound effect on ecosystems
Lesson 2.1.4 - Introduction to Biological Polymers
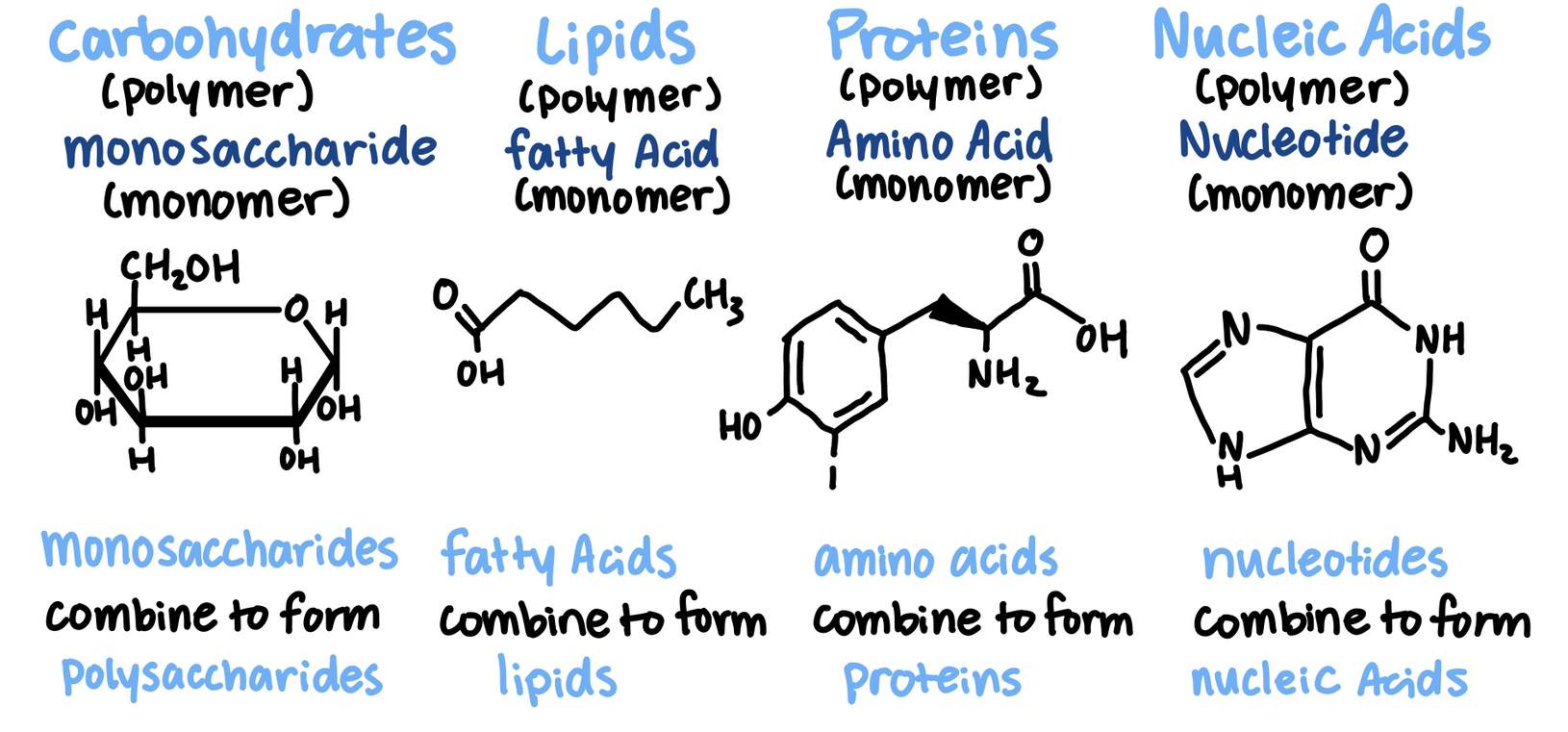
All living things are made of 4 classes of large biological molecules: carbohydrates, lipids, proteins, nucleic acids
- Biological polymers are formed by the linking together of smaller organic molecules known as monomers
Myoglobin is an iron- and oxygen-binding protein in the muscle tissue of mammals
- Myoglobin is a long, single chain (polymer) of about 150 individual monomer units called amino acids
Carbon is unique in its ability to form the skeletons of large, complex, diverse molecules that are necessary for life’s functions.
Organic carbon compounds → Basis of the chemistry of life (may contain carbon, hydrogen, oxygen, nitrogen)
Inorganic carbon compounds → (may contain carbon, oxygen, nitrogen)
The simplest organic compounds are hydrocarbons → made of only carbon + hydrogen
- Methane (CH4) is the simplest hydrocarbon molecule
- Larger hydrocarbons (like those in fats) serve as important fuels in the body
- Organic compounds have unique 3D shapes and living organisms rely on their ability to recognize the shape
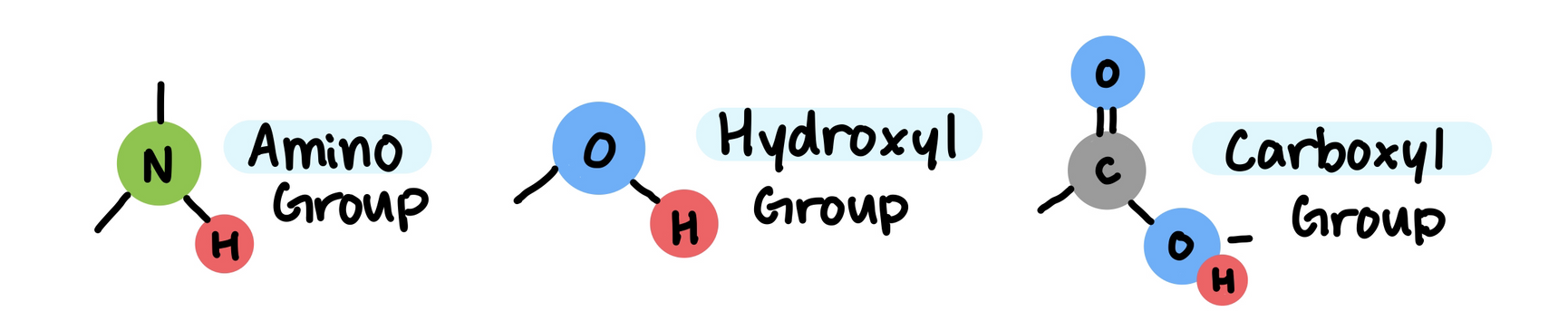
Functional Groups
The properties of an organic compounds depend on its carbon skeleton and the atoms attached to it.
These atoms (that usually participate in chemical reactions) are called functional groups
Lesson 2.1.5 - Carbohydrates
Used by cells as fuel and building material
Includes sugars and polymers of sugars
- Monosaccharides link together to form polysaccharide
Monosaccharides have molecular formulas that are usually multiples of CH2O
Glucose (C6H12O6) is the most common monosaccharide
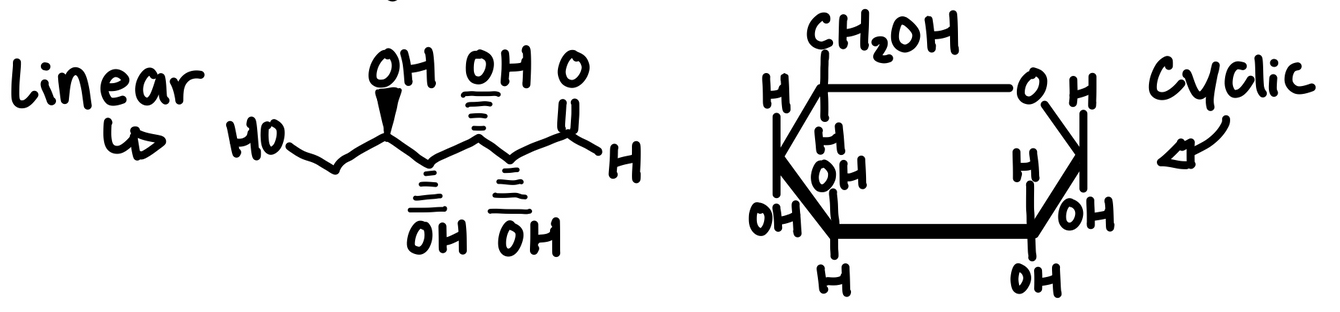
Monosaccharides serve as fuel and building material for cells
Two monosaccharides can react to form a disaccharide by forming a glycosidic bond
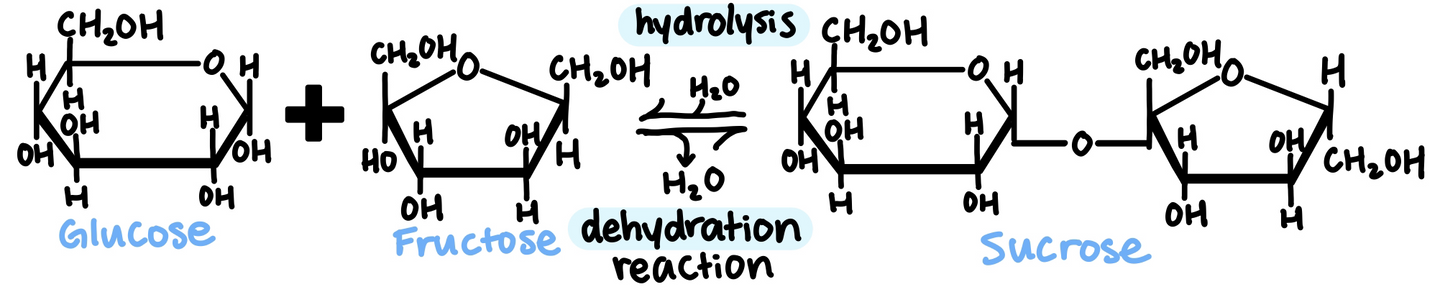
Polysaccharides serve both storage and structural roles in organisms
- cellulose and chitin form the structures of plants and insects
- In plants, the polysaccharide molecule used for fuel storage is amylose (starch) and in animals, is typically stored as glycogen
A polysaccharide’s unique 3D shape relates to its formations of glycosidic bonds and hydrogen bonding
- Glycogen has a helical structure, cellulose has a linear structure
Cellulose is indigestible because the linear molecules pack closely together in a large network held together by hydrogen bonding. Cellulose has few positions available for hydrogen bonding with water molecules, making cellulose insoluble in water.
- This insolubility means that animals are unable to break cellulose down for fuel
- This property makes it excellent material for structures, such as tree bark and cell walls, where it is important to have a water impenetrable barrier.
Lesson 2.1.6 - Lipids
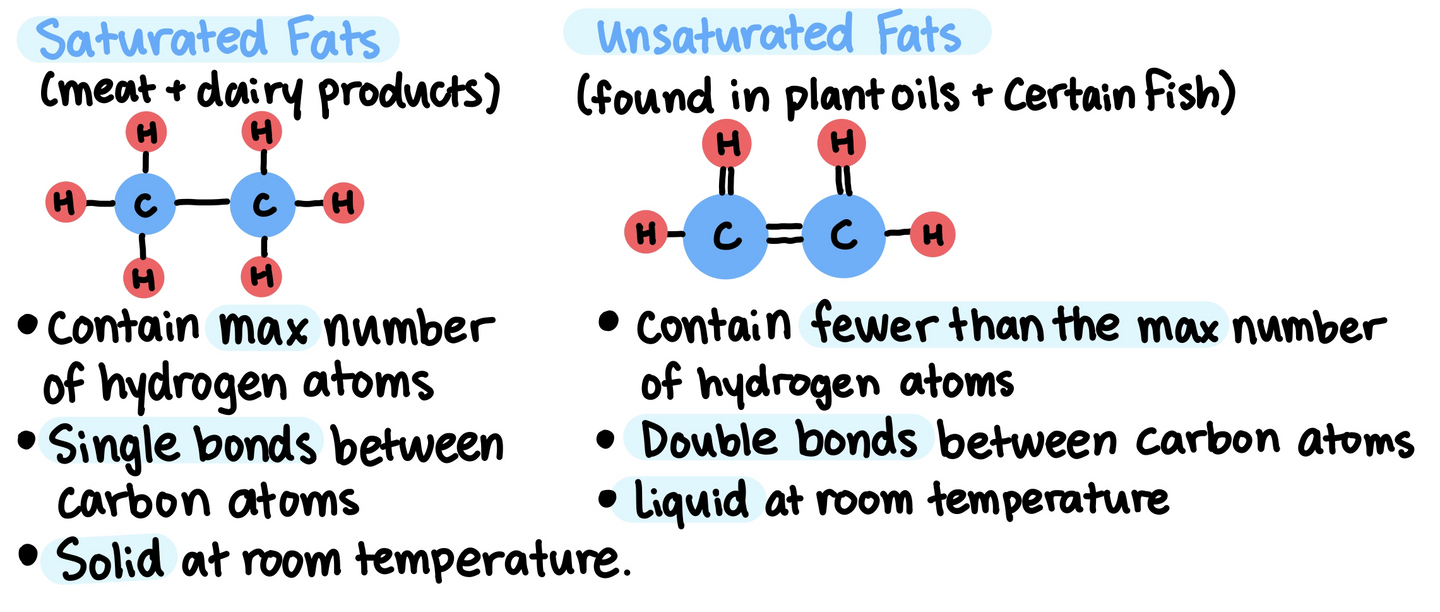
Lipid molecules are generally hydrophobic (unable to hydrogen bond with water and other aqueous molecules)
- Lipids are insoluble in water because they are mostly composed of hydrocarbons that form non-polar covalent bonds.
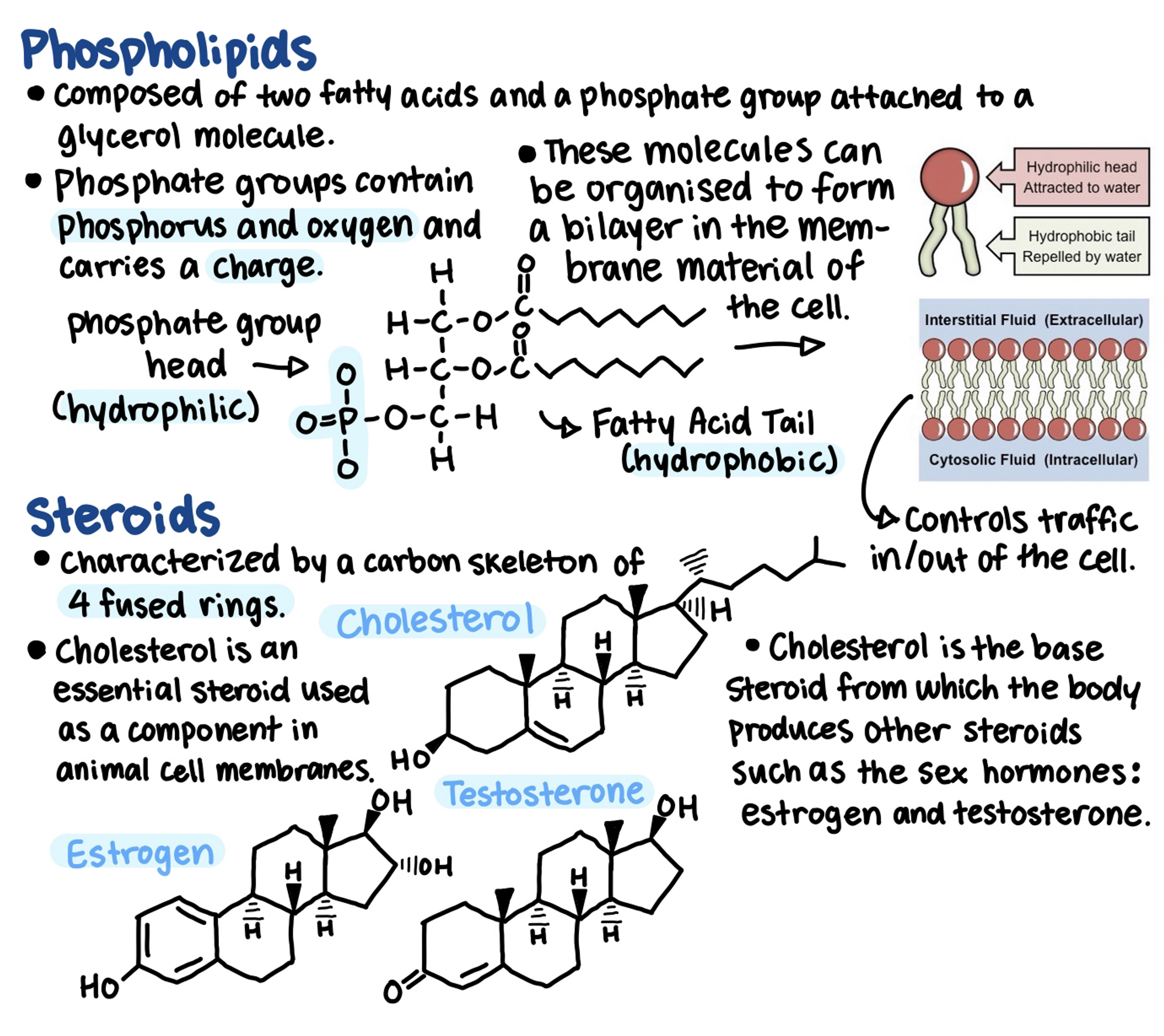
- Biologically important lipids include fats, phospholipids, steroids
Fats differ in structure to carbohydrates, proteins, and nucleic acids because fats are not true polymers.
Fats are not long chains made up of the same repeating unit, they are made up from 2 kinds of smaller molecules:
- Glycerol + Fatty Acids
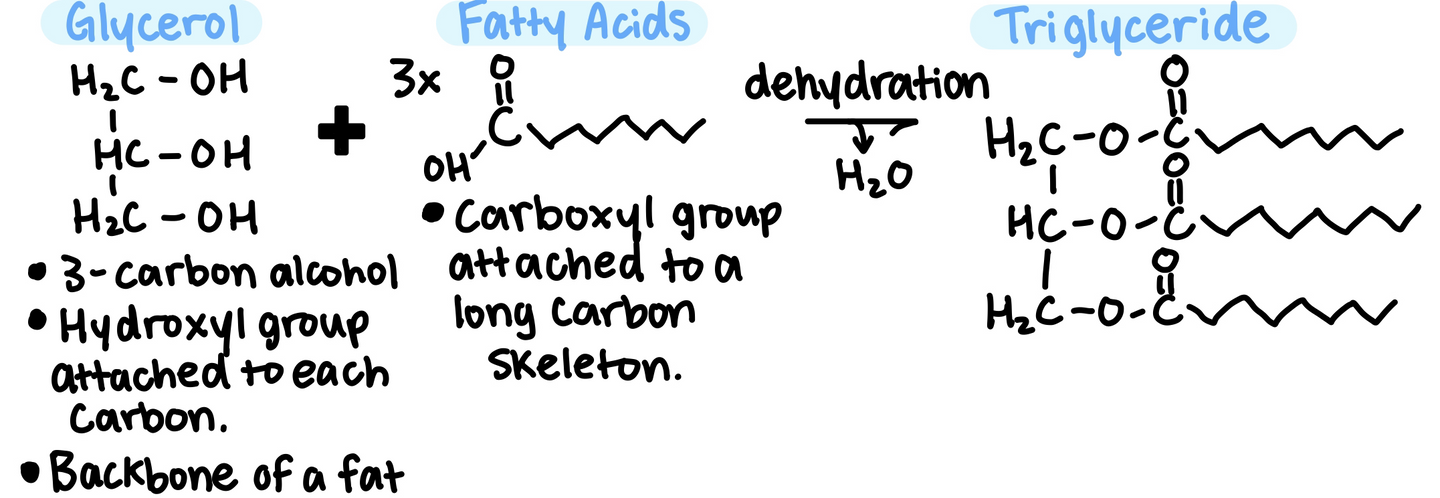
The long carbon chain stores a lot of energy, which is one reason why the body stores fat for later use as fuel.
Phospholipids are composed of 2 fatty acids and a phosphate group attached to a glycerol molecule.
Lesson 2.1.7 - Proteins
Proteins have many shapes, sizes, and structures that perform a range of functions:
- Catalyzing biochemical reactions
- Structural support
- Storage
- Transport
- Communication
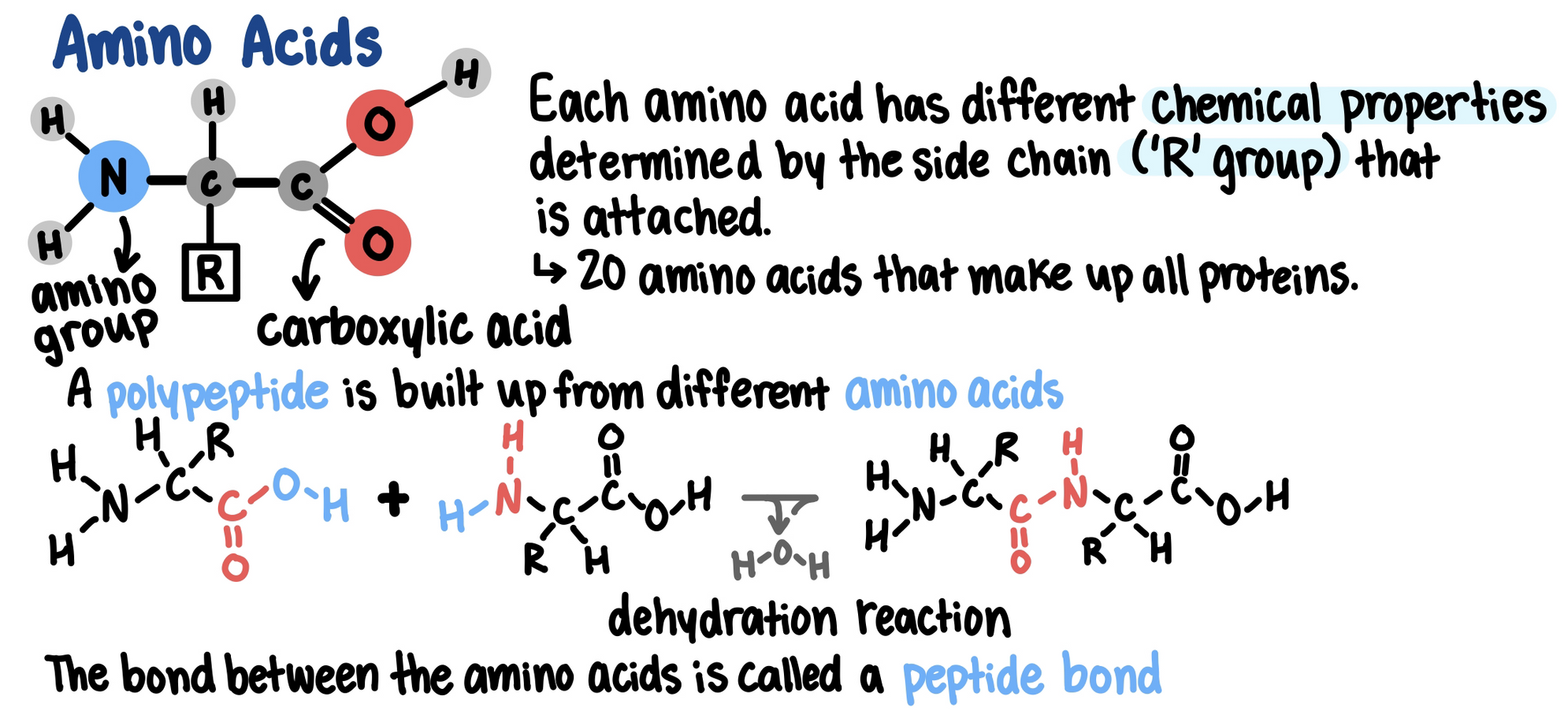
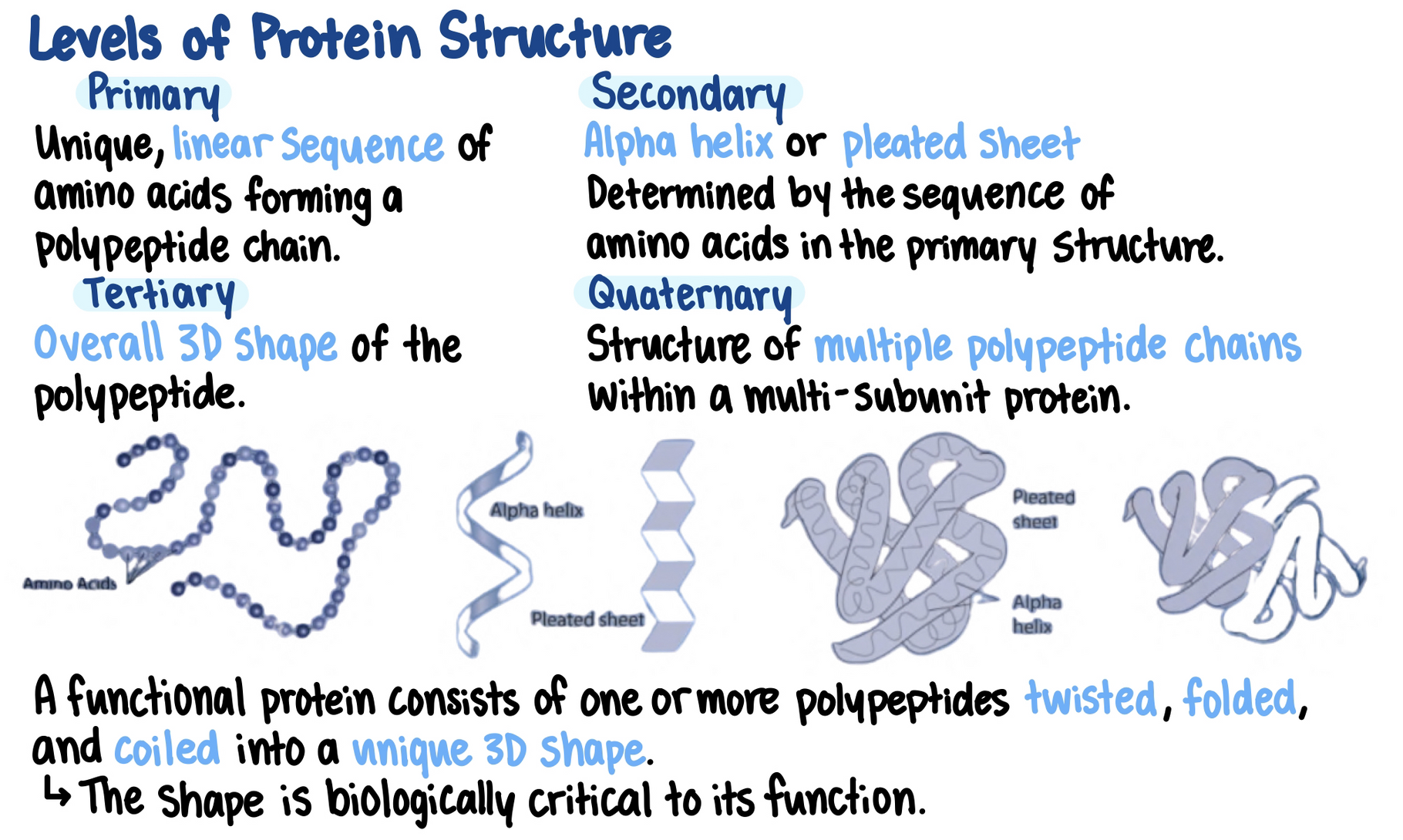
Proteins are made of one or more polypeptides (polymers)
Polypeptides are made of multiple amino acids (monomers)
Keratin → Hair and fingernails (structural)
Osteopontin → Eggshells (storage)
Hemoglobin → In red blood cells (transport)
Each protein has a unique primary structure that enables it to do a specific job within a cell
Misfolded proteins are associated with many diseases including nervous system disorders (e.g. Alzheimer’s disease, Bovine Spongiform Encephalopathy, Parkinson’s disease, etc.)
The primary structure of a protein (its sequence of amino acids) cause it to fold into its functional shape.
- The amino acid sequence is specified by a gene
Lesson 2.1.8 - Nucleic Acids
Nucleic acids are macromolecules that provide directions for building proteins.
The role of nucleic acids is to store and transmit hereditary information.
DNA (Dioxyribonucleic acid)
- Resides in a cell as long fibres called chromosomes
- Stretches of DNA called genes program the amino acid sequence of a polypeptide
RNA (Ribonucleic acid)
- Translate nucleic acid language into protein language
Nucleic acids are polynucleotides (polymers) made of many nucleotide building blocks (monomers)
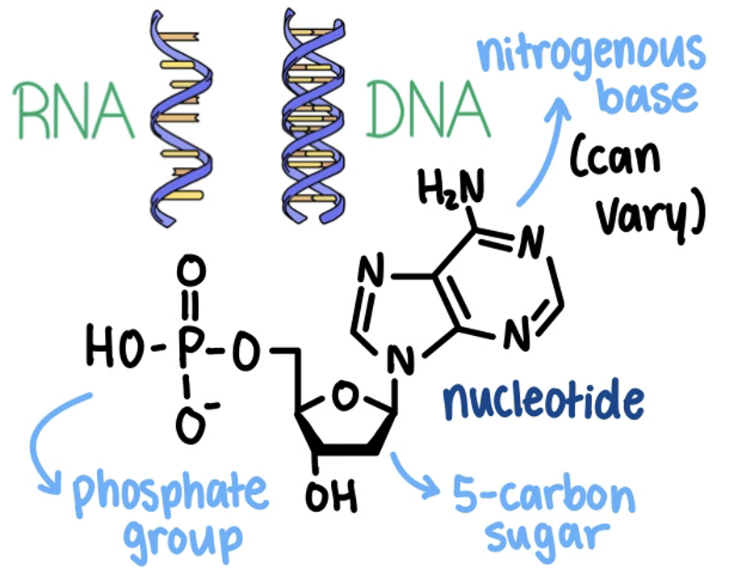
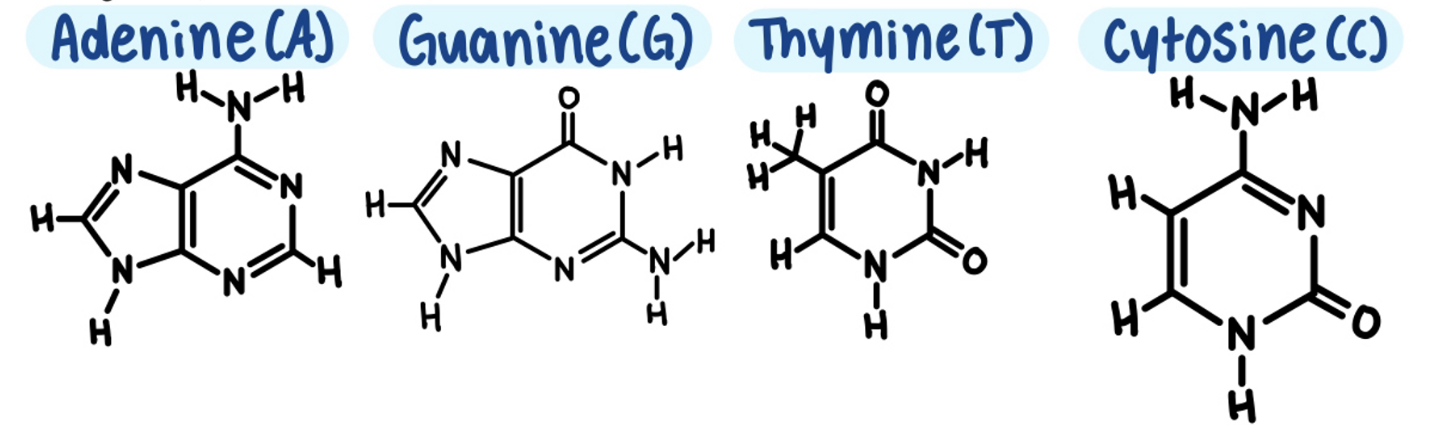
Each DNA nucleotide has one of 4 nitrogenous bases.
All genetic information is written using only these 4 bases