gen. chem II exam 1 content complete
1/64
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
65 Terms
Condensation
the transition from gases to liquids (exothermic reaction)
Vaporization
the transition from liquids to gases (endothermic reaction)
rate of condensation = rate of vaporization
liquid and gas don’t change
vapor pressure
pressure exerted by vapor with a liquid in a closed container
relation between vapor pressure and IMFs/KE
as temperature increases, _____ increases due to increase KE (breaking IMFs)
boiling point
temperature where the vapor pressure = external pressure / atmospheric pressure
normal boiling point
the boiling point of a liquid when surrounding P = 1atm
endothermic reaction
moving from a low energy state to a higher energy state (absorbing heat from surroundings)
exothermic reaction
moving from a high energy state to a lower energy state (releasing heat into surroundings)
enthalpy of vaporization
amount of heat energy required to convert one mole of a liquid into a gas at constant pressure
enthalpy of vapor pressure and IMFs
higher vapor pressure = weaker IMFs
melting
phase transition from solid to liquid (endothermic) [temp stops rising even if heat is added, until all of the solid is _____, then it will change.]
freezing
liquid to solid (exothermic)
Melting Point of Solid / Freezing Point of Liquid
the temperature at which the solid and liquid phases are in equilibrium
enthalpy of fusion
amount of heat needed to change one mole of a substance from solid state to a liquid state (melting)
enthalpy of freezing
amount of heat needed to change one mole of a substance from liquid state to a solid state
sublimation
phase transition from solid to gas (endothermic)
deposition
phase transition from gas to solid (exothermic)
phase energy levels
solid < liquid < gas
enthalpy of sublimation
amount of heat needed to change one mole of a substance from solid state to a gaseous state
enthalpy of sublimation equation
△Hsub = △H,fusion + △H,vaporization
enthalpy relations
△H,fusion < △H,vap < △H,sub
heat and IMFs
higher heat = weaker IMFs (being overcome)
q = mc△T
heat equation with no change in phases
q = n△Hrxn
equation that represents heat needed to induce a change in phases
phase diagram
combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase transition equilibria of a substance
also indicates physical states that exist under specific conditions (P & T) and transition temperatures
triple point
the temperature and pressure where solid, liquid and gas phases coexist in equilibrium
critical point
the point in temperature and pressure where the liquid and gaseous phases of a substance merge together in a single phase
supercritical fluid
combination of liquid and gas properties.
Molecular Orbital (MO) Theory
explains chemical bonds by considering electrons to be delocalized over an entire molecule rather than confined to individuals AOs. AOs combine to make MOs.
Atomic Orbital (AO) theory
describes electrons in individual atoms rather than the molecules, explains how electrons are distributed in AOs and how the AOs define an atom’s shape
Orbital
A region in an atom where there is high probability of finding an electron. represents the spatial distribution of an electron described by quantum numbers
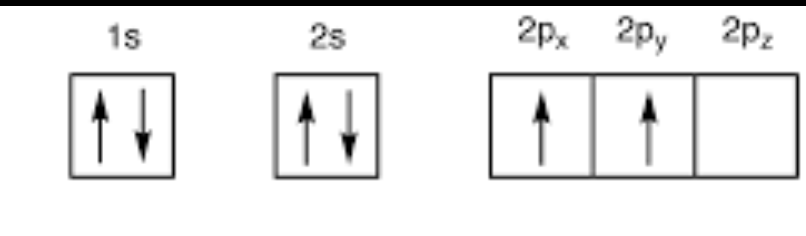
excited state
a condition in an atom or molecule where one or more electrons have absorbed energy and moved to a higher energy level than their normal "ground state,
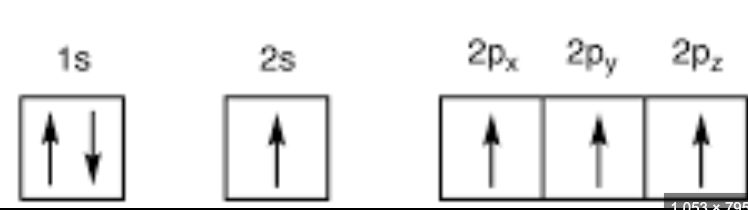
ground state
the arrangement of electrons in an atom where all electrons occupy the lowest possible energy levels, representing the most stable and lowest energy state of the atom;
Valence Bond (VB) theory
explains chemical bonding by assuming that a bond forms when AOs of two atoms overlap, electrons are shared, and each atom retains its own AO, but the region in which they overlap merges. the strength of these bonds depend on the extent of the overlap (the greater the overlap, the stronger the bonds [sp3-s > sp-s])its
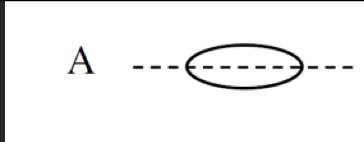
σ2s
σ*2s
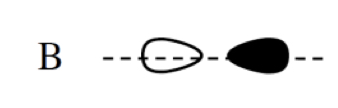
bonding MO
type of orbital formed when atomic orbitals from different atoms overlap constructively, resulting in increased electron density between the nuclei and creating a stable chemical bond between the atoms
more stable than anti bonding
has constructive interference (in-phase)
higher electron density in the internuclear region (constructive interference)
antibonding MO
a molecular orbital that increases the energy of a molecule and weakens the bond between two atom
higher energy than bonding due to e- repelling
has destructive interference (out-of-phase)
has a region of zero electron density at the node
σ2p
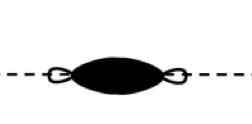
σ*2p
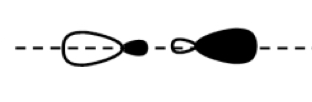
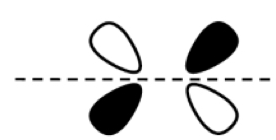
π2p

π*2p
π2s or π*2s
DOES NOT EXIST
paramagnetic v. diagmagnetic
paramagnetic has an unpaired electron; diamagnetic does not
bond order of zero
there are no bonds between the two atoms involved, essentially indicating that a molecule cannot form between them as there is no net attraction holding them together
electron density
refers to the probability of finding an electron at a specific point in space around an atom or molecule, essentially representing the "concentration" of electrons in a given area
σ framework
π framework
in-phase
the interaction between two atomic orbitals where their wave functions overlap constructively, meaning their phases align (both positive or both negative). This results in an increase in electron density between the nuclei, leading to the formation of a lower-energy, more stable bonding molecular orbital.
out-of-phase
the interaction between two atomic orbitals where their wave functions overlap in a way that creates a node between the nuclei, resulting in a higher energy, less stable "antibonding" molecular orbital
sigma and pi bond relation
a single bond is a sigma bond, a double bond is one sigma and one pi, a triple bond is one sigma and two pi bonds.
head-head overlap
when the ends of atomic orbitals from two atoms overlap, forming a sigma bond
side-to-side overlap
overlap of atomic orbitals which is a π bond, It's a type of covalent bond that occurs when two p orbitals overlap
Hydrogen bonding
a special type of dipole-dipole force that occurs when H is bonded to F, O, or N (FON); strongest bond among IMFs, which is why water has a high boiling point than larger molecules w/ only dispersion forces.
dipole-dipole forces
attraction between positing end of one polar molecule and the negative end of another polar molecule; weaker than hydrogen bonds but stronger than dispersion forces.
dispersion forces
weak, temporary attractions among all IMFs, but can be strong in large surface area molecules. found in all molecules, but they are the only IMF present in non-polar molecules (e.g, hydrocarbons)
boiling point (Tb)
the temperature at which a liquids vapor pressure (Pvap) equals external/atmospheric pressure (Pext), causing the liquid to turn into gas
stronger IMFs = higher _____ b/c more energy is needed to separate molecules (H2O has high Tb due to H-bonding)
vaporization
general process of liquid turning into gas. faster at higher temperatures.
weaker IMFs = faster _____ b/c molecules can break bonds and escape more easily.
viscosity
resistance for a liquid to flow (high _____ = honey).
stronger IMFS = higher ______ b/c molecules will stick together.
Larger molecules = higher _____ b/c more dispersion forces
capillary action
the ability of a liquid to flow against gravity in a narrow tube. it depends on cohesive and adhesive force
Stronger IMFs if adhesion > cohesion
common non-polar molecular geometric shapes (NOT ALWAYS THOUGH)
linear, trigonal planar, and tetrahedral
_____ molecules (BF3 = trigonal planar) (CCl4 = tetrahedral) (CO2 = linear)
common polar molecular geometric shapes (NOT ALWAYS THOUGH)
t-shaped and see-saw
______ molecules (BrF3 = T-shaped) (SF4 = see-saw)
constructive interference
occurs when two wave functions overlap in phase and create a larger amplitude, in MO theory it leads to the formation of bonding molecular orbitals, which have lower energy and greater stability.
destructive interference
occurs when two wave functions overlap out of phase .This results in a decrease or complete cancellation of the wave amplitude. In MO theory, this leads to the formation of antibonding molecular orbitals, which have higher energy and reduced stability due to the presence of a node between nuclei.