PMT 3.3 - Haloalkanes Flashcards
Are halogenoalkanes soluble in water?
Are halogenoalkanes soluble in water?
Insoluble as C-H bonds are non-polar, not compensated for enough by C-X bond polarity
Do halogenoalkanes have a polar bond? why?
Do halogenoalkanes have a polar bond? why?
Yes polar, as halogen has a higher electronegativity than C ( halogen is δ-, carbon is δ+)
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
Are halogenoalkanes soluble in water?
Are halogenoalkanes soluble in water?
Insoluble as C-H bonds are non-polar, not compensated for enough by C-X bond polarity
Do halogenoalkanes have a polar bond? why?
Do halogenoalkanes have a polar bond? why?
Yes polar, as halogen has a higher electronegativity than C ( halogen is δ-, carbon is δ+)
Which intermolecular forces do they have? why?
Which intermolecular forces do they have? why?
Permanent dipole-dipole and van der Waals forces of attraction
C-X bond polarity creates permanent dipoles
When would they have higher boiling points?
When would they have higher boiling points?
Increase Carbon chain length
Halogen further down group 7
How would the mass of a haloalkane compare with the mass of an alkane of the same chain length?
How would the mass of a haloalkane compare with the mass of an alkane of the same chain length?
Greater as mass of halogen > mass of H
What is the most important factor in determining their reactivity?
What is the most important factor in determining their reactivity?
Carbon-halogen bond enthalpy
What would bond polarity suggest the order of reactivity would be?
What would bond polarity suggest the order of reactivity would be?
C-F would be most reactive as most polar bond
What would bond enthalpies suggest the order of reactivity would be?
What would bond enthalpies suggest the order of reactivity would be?
C-I would be most reactive as lowest bond enthalpy
What is a nucleophile?
What is a nucleophile?
electron pair donor
Give 3 examples of nucleophiles
Give 3 examples of nucleophiles
:OH-
:CN-
:NH3
What is nucleophilic substitution?
What is nucleophilic substitution?
A reaction where a nucleophile donates a lone pair of electrons to δ+ C atom, δ− atom leaves molecule (replaced by nucleophiles)
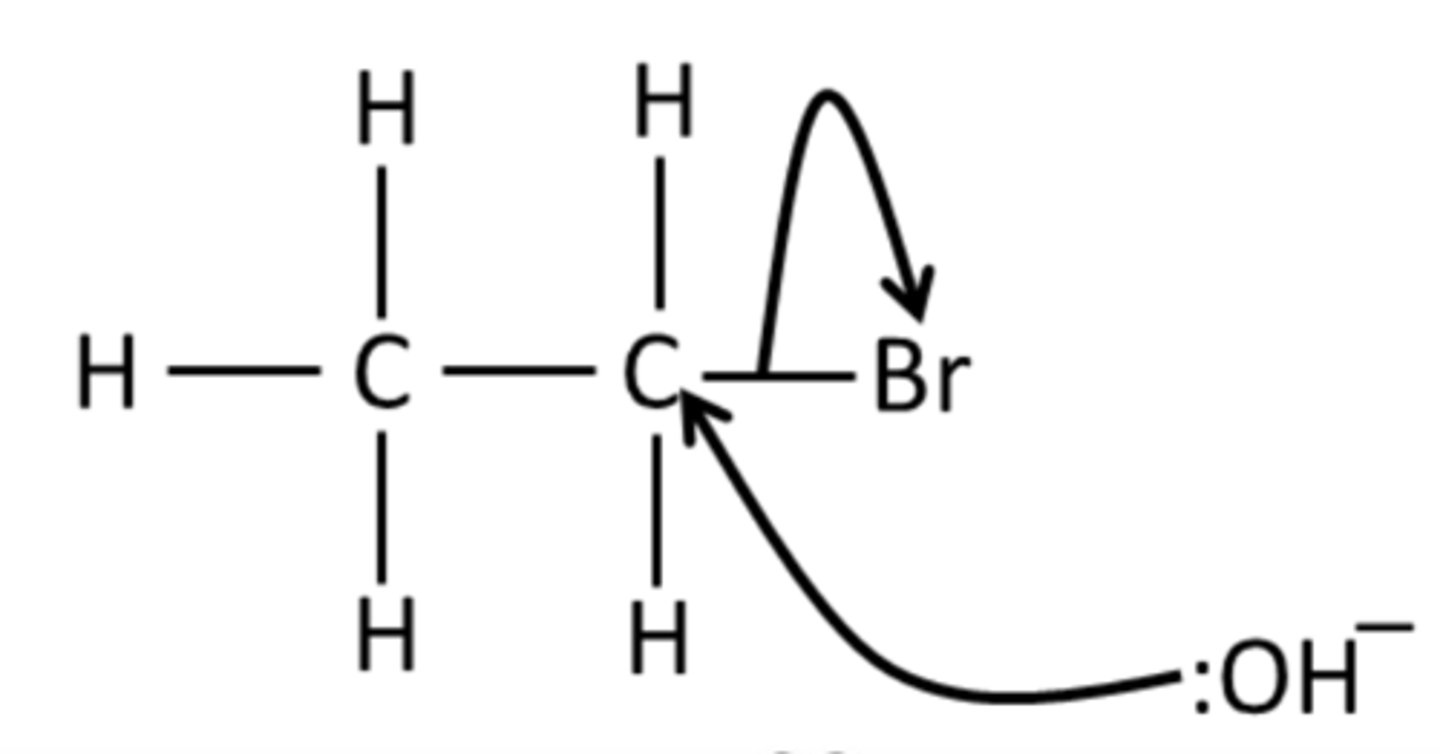
Draw the mechanism for the reaction of bromoethane with NaOH (aq).
Draw the mechanism for the reaction of bromoethane with NaOH (aq).
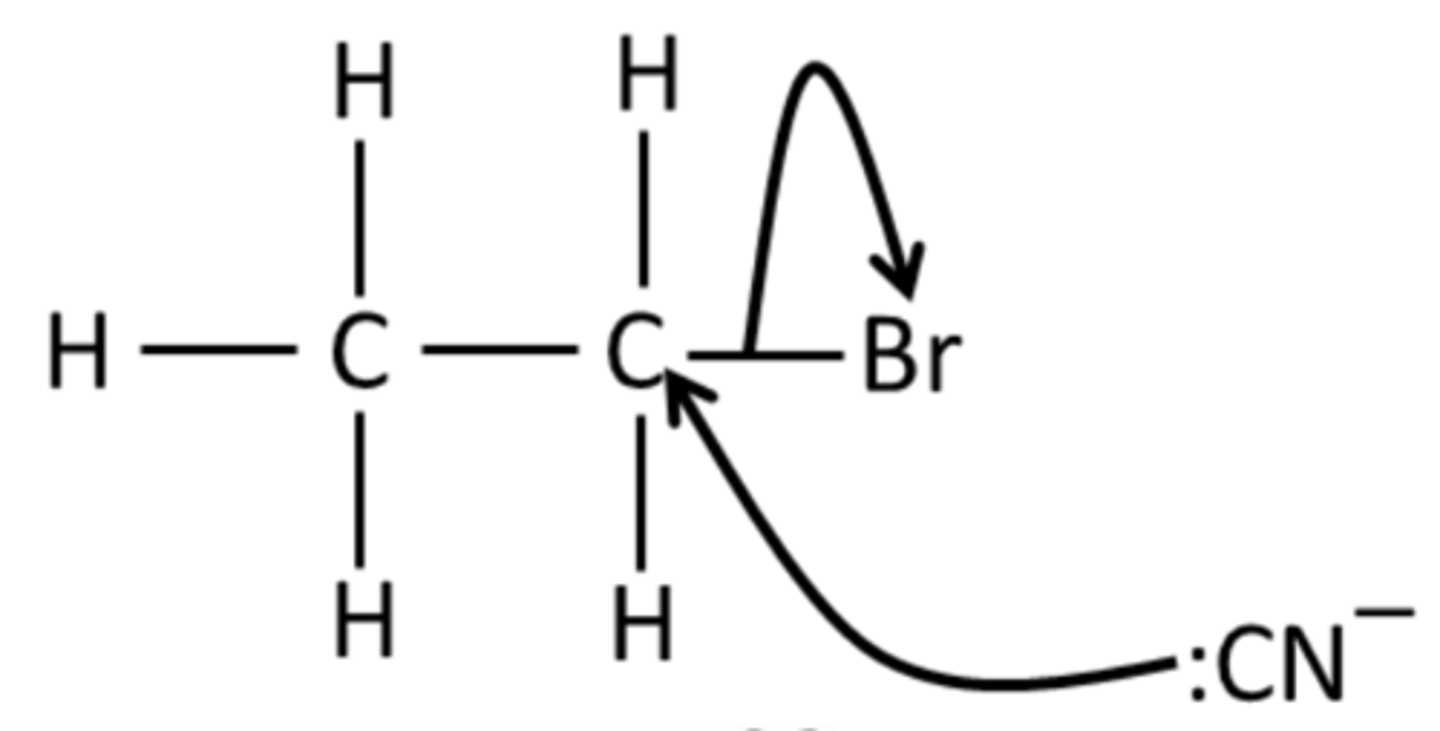
Draw the mechanism for the reaction of bromoethane with KCN
Draw the mechanism for the reaction of bromoethane with KCN
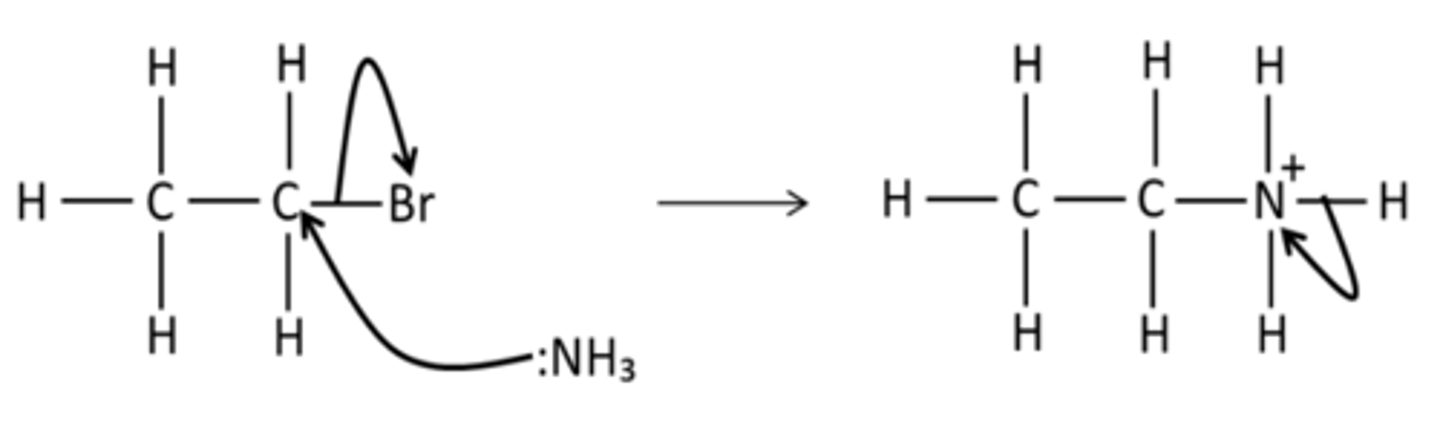
Draw the mechanism for the reaction of bromoethane with NH3
Draw the mechanism for the reaction of bromoethane with NH3
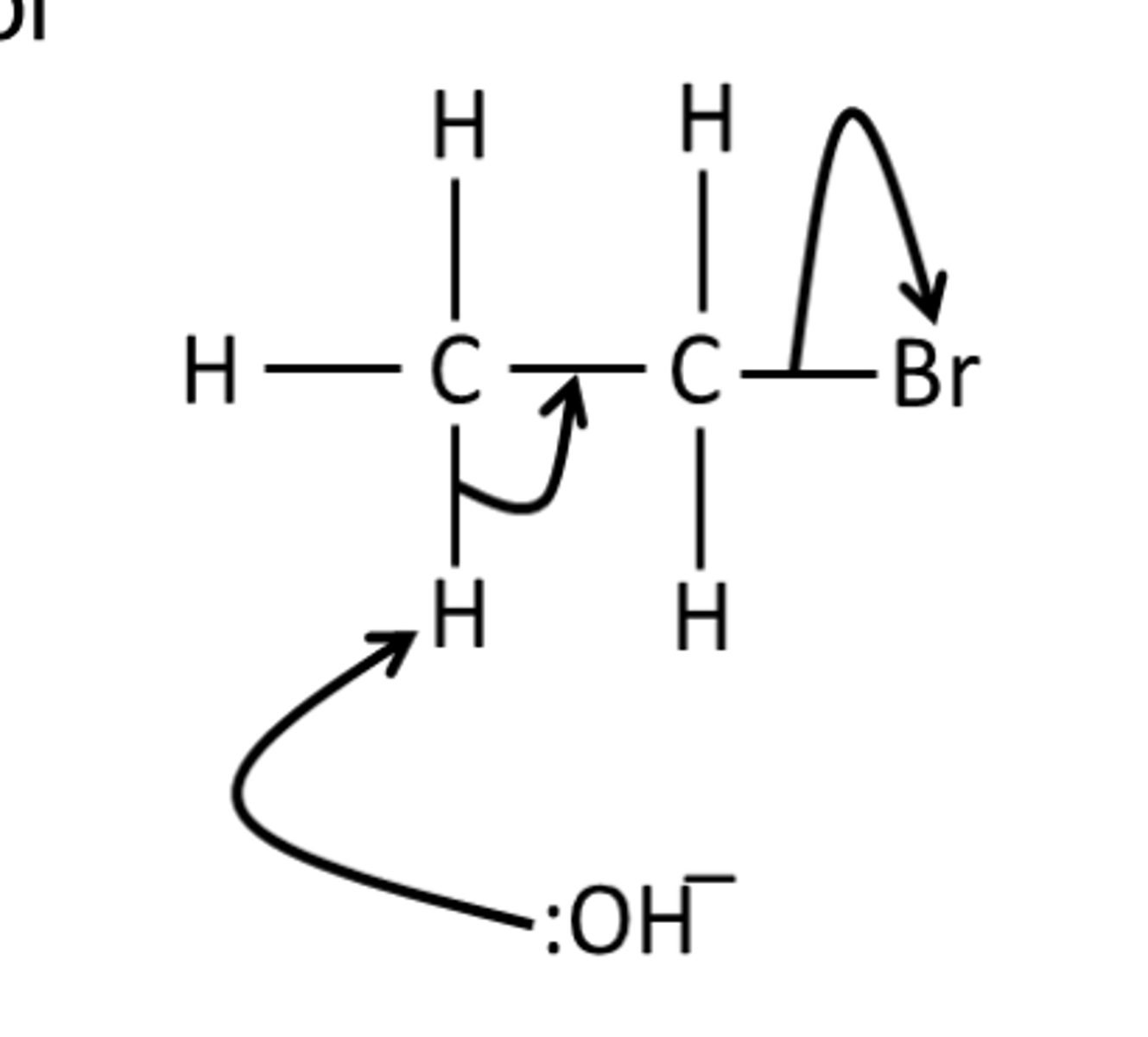
Draw a mechanism for the reaction of bromoethane with NaOH in ethanol
Draw a mechanism for the reaction of bromoethane with NaOH in ethanol
What are CFCs?
What are CFCs?
Chlorine-fluoro-carbons - haloalkanes containing C, F and Cl only (no H)
What is the problem with CFCs?
What is the problem with CFCs?
Although unreactive under normal conditions, they catalyse the breakdown of ozone in the atmosphere via free radical substitution
What are CFCs being replaced with?
What are CFCs being replaced with?
HCFCs (hydrogen, chlorine, fluorine, carbon)
HFCs (hydrogen, fluorine, carbon)
What are the conditions/ reactants needed for the elimination reaction of haloalkanes?
What are the conditions/ reactants needed for the elimination reaction of haloalkanes?
NaOH or KOH dissolved in ethanol (no water present)
Heated
What is formed in the elimination reaction of haloalkanes?
What is formed in the elimination reaction of haloalkanes?
An alkene, water and halogen ion