1.1 Atomic Structure (Booklet 1 of 8)
0.0(0)
Card Sorting
1/8
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
1
New cards
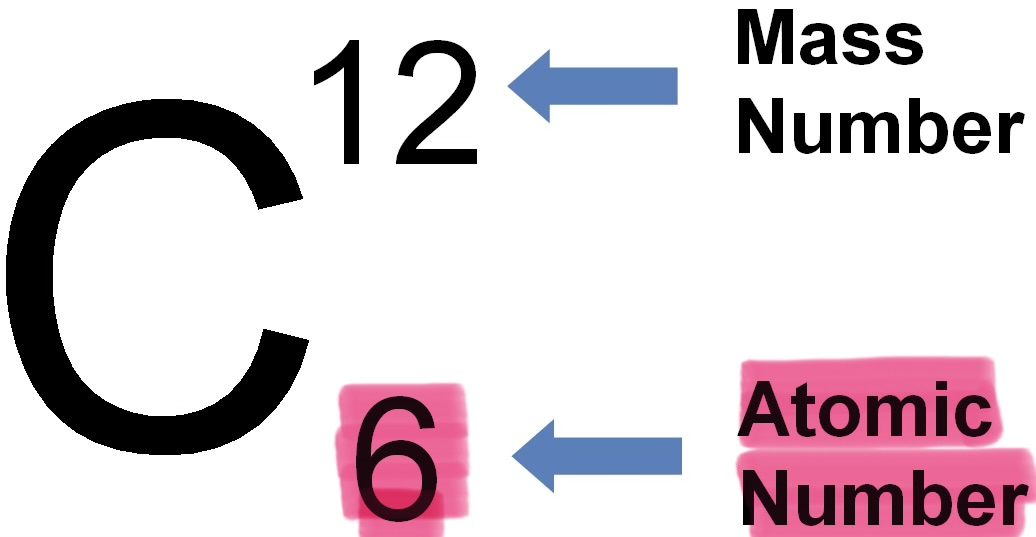
Atomic number
The number of protons in the nucleus of an atom.
2
New cards
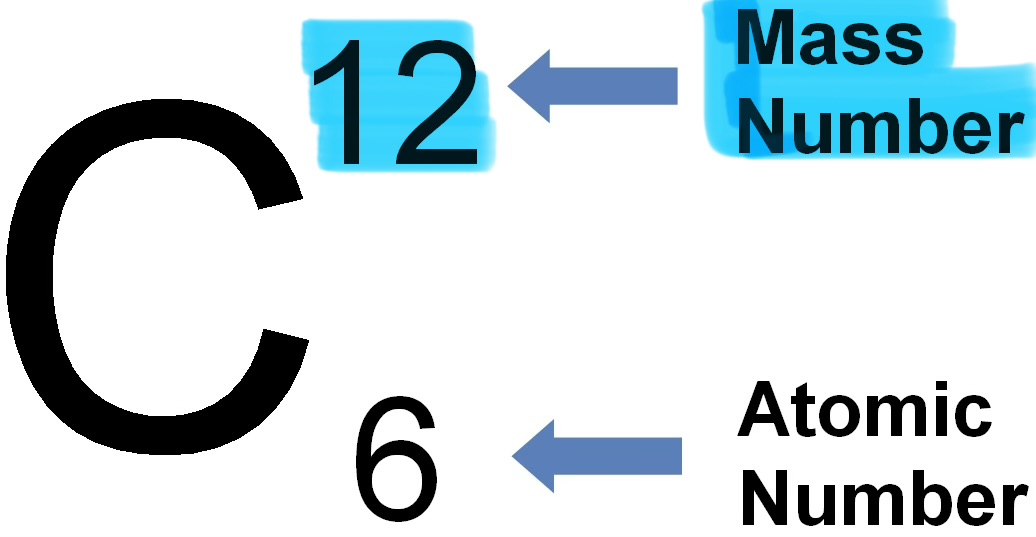
Mass number
The total number of protons and neutrons in the nucleus of an atom.
3
New cards
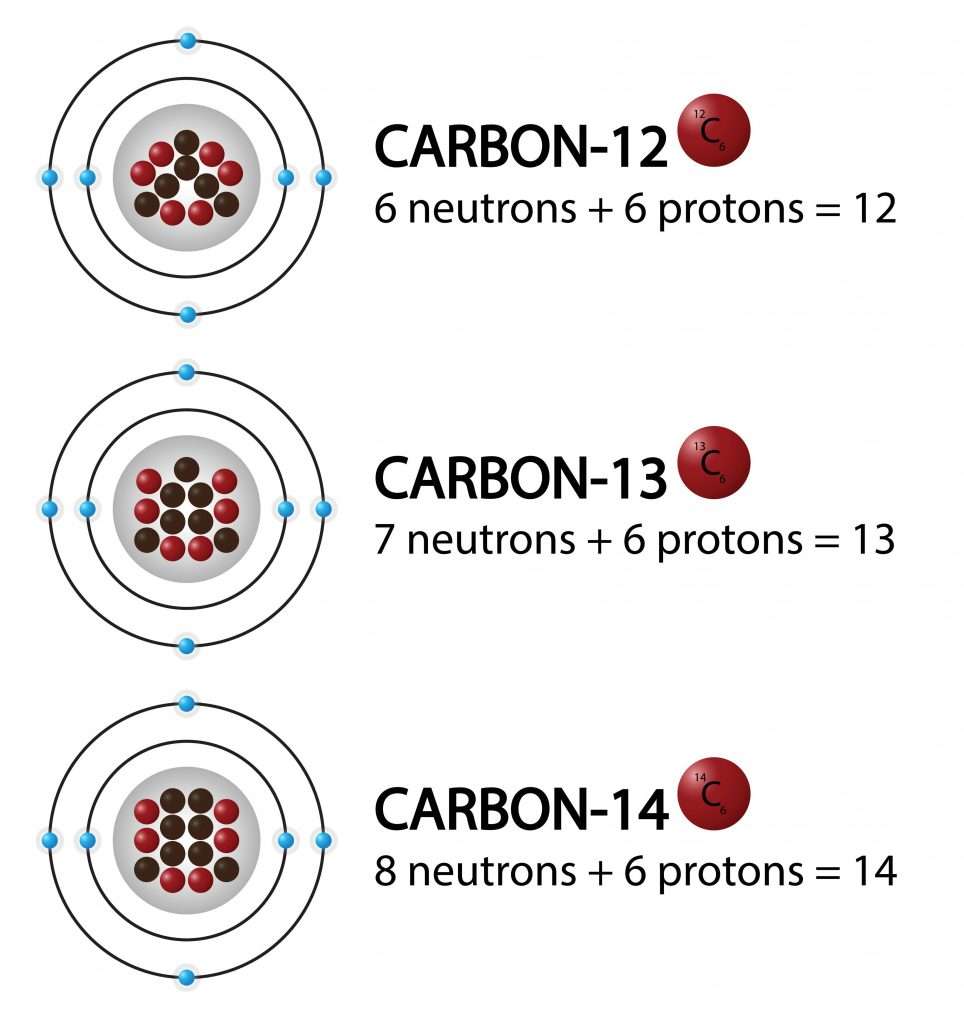
Isotopes
Isotopes are atoms of an element with the same atomic number but a different mass number.
4
New cards
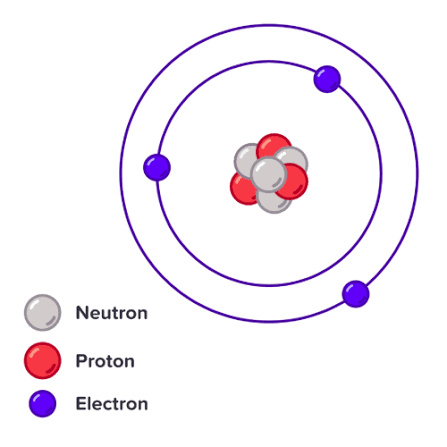
Ion
A charged particle formed when an atom gains or loses electrons.
5
New cards
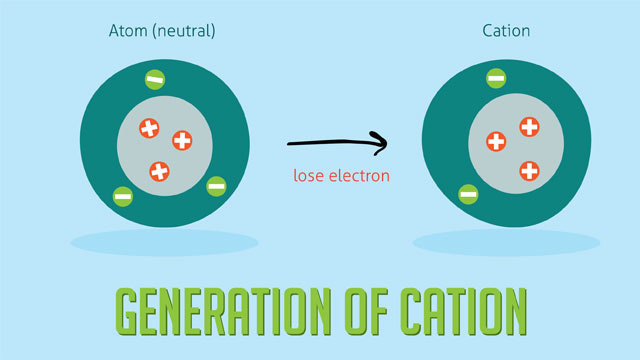
Cation
A cation is a positive ion.
6
New cards
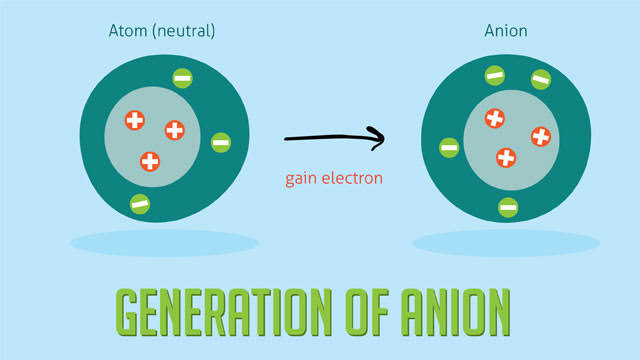
Anion
An anion is a negative ion.
7
New cards
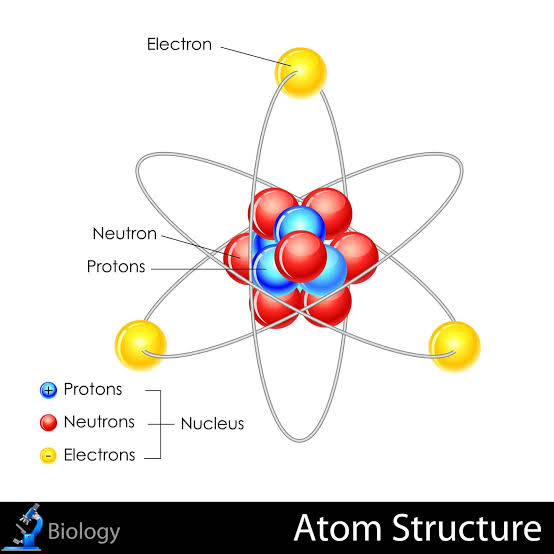
Atom
The simplest particle of an element that can exist on its own in a stable environment.
8
New cards
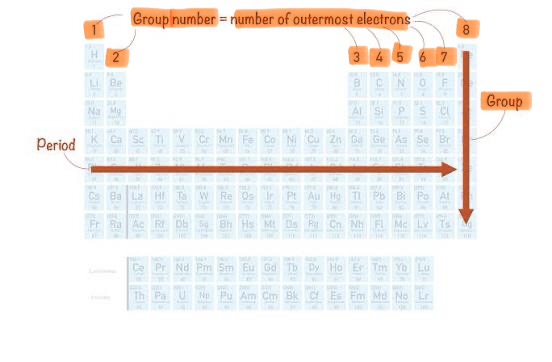
Group
Vertical column in the periodic table.
9
New cards
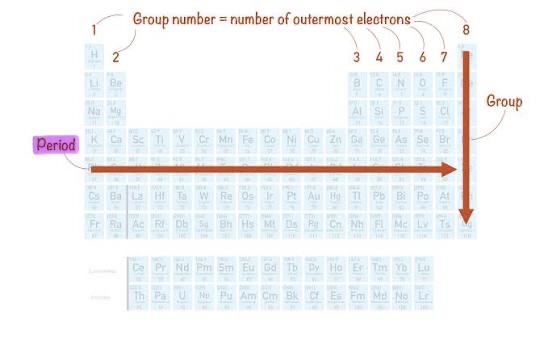
Period
Horizontal row in the periodic table.