KMCAT 1 Biology Amino Acids & Enzymes
1/60
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
61 Terms
Phosphorylation
Addition of phosphate groups (usually to serine, threonine, or tyrosine), playing a critical role in signaling pathways.
Glycosylation
Attachment of carbohydrate groups, affecting protein folding, stability, and cell recognition
Acetylation and Methylation
Modifications that typically occur on lysine residues, influencing gene expression and protein function
Ubiquitination
Attachment of ubiquitin to lysine residues, tagging proteins for degradation
Amino acids such as tryptophan, tyrosine, and phenylalanine, which have large ring structures in their R groups, are often found in __, perhaps because this structure provides plenty of space for the side chains
beta pleated sheets
If proteins can go from unstructured to folded all by themselves, their amino acid sequences __ contain all the information needed for folding.
do
Hydrophobic residues clustering together __ the entropy of water compared to when the residues were individual and not clustered together.
increase
Chaperonins
These are large, cylindrical complexes that provide a protected environment for protein folding. A well-known example is the GroEL/GroES system in bacteria. This isolation prevents aggregation and allows the protein to fold properly within the chamber. After folding, the protein is released, and the chaperonin complex is ready to assist with another substrate.
Molecular chaperones
These proteins bind to nascent or partially folded polypeptides, preventing improper interactions that can lead to aggregation or misfolding.
The _ form of amino acids is the only type found in the human body and it has the amino group on the _ side.
L, left
Acid hydrolysis cleaves at _ amino acid(s) and protyolysis cleaves at _ amino acid(s).
each, specific
pH < pKa = _
pH > pKa = _
protonated, deptrotonated
Two amino acids, _ and _ break alpha helixes because the first introduces kinks into the helix and the second is flexible around its bonds because its achiral, which allows it to break the helix structure.
proline, glycine
cysteine = _ form
cystine = _ form
reduced (intracellular, single), oxidized (extracellular, disulfide bonded)
Whats the isoelectric point?
The pH at which a molecule carries no net charge.
For amino acids, it’s the pH where the molecule is zwitterionic: the amino group is protonated (NH₃⁺) and the carboxyl group is deprotonated (COO⁻), and any other ionizable groups are balanced.
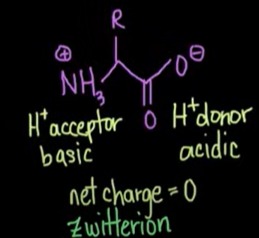
Denaturation does not change the _ structure of an amino acid.
primary
aliphatic
non-aromatic
At physiologic pH, basic functional groups will be __, whereas acidic functional groups will be __.
protonated, deprotonated
Is the COOH of an amino acid acidic or basic?
Is the amino group of an amino acid acidic or basic?
acidic, basic
What is a zwitterion?
a neutral molecule that contains both a positive and negative charge
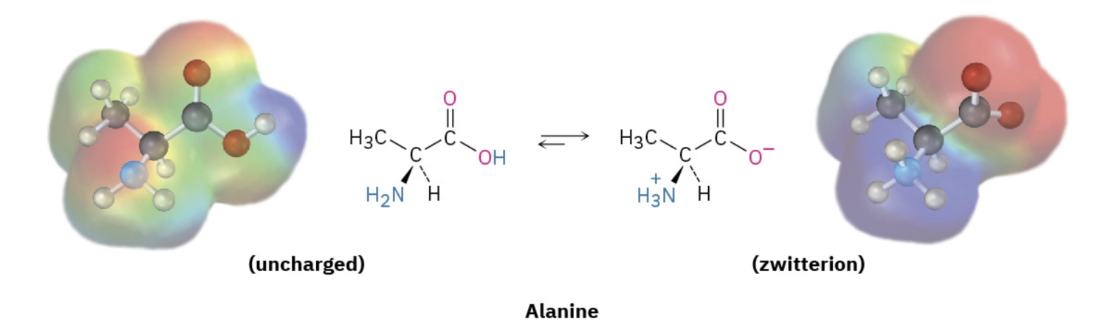
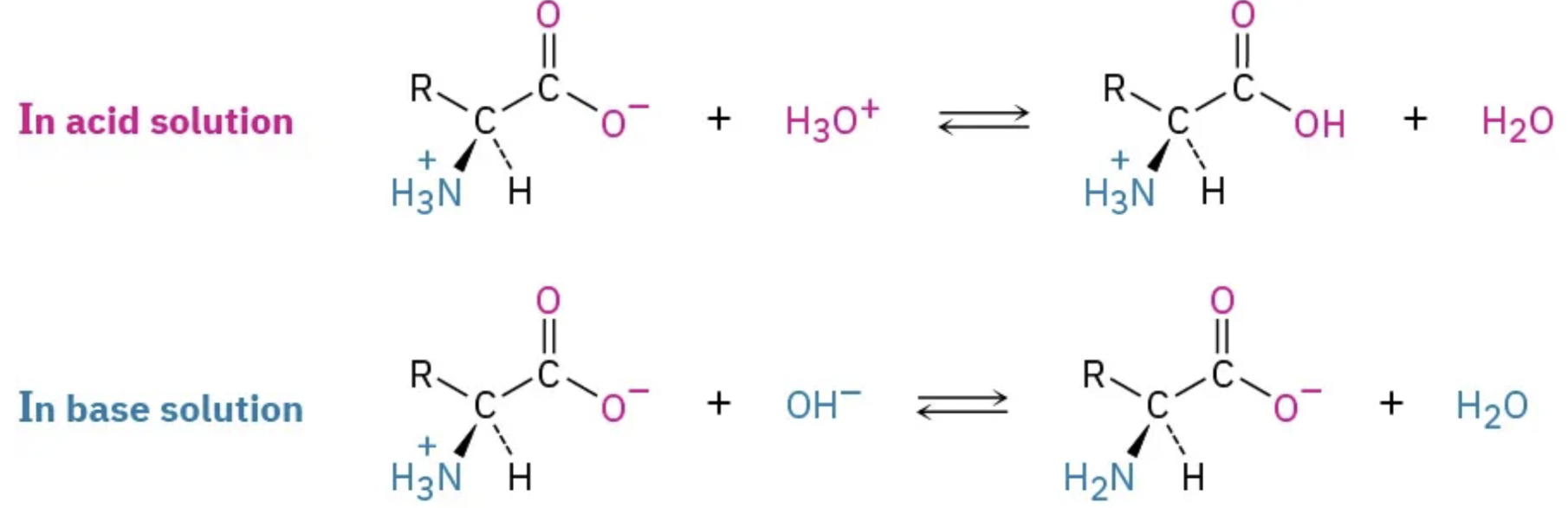
How do the amino and carboxyl groups of an amino acid act in acidic and basic conditions? What are their charges?
Native vs SDS Page
Native: Proteins are separated based on their native conformation and charge.
SDS: Proteins are denatured and separated based on their molecular weight.
Which way do proteins move in Native and SDS PAGE?
For SDS: anode, +, because proteins are negatively charged naturally and SDS Page coats all the proteins with an equal mass to negative charge
For Native: to the side opposite the protein’s charge
Two types of SDS Page
reducing conditions: disulfide bonds between cysteine residues are broken. This disrupts the quaternary structure of proteins that consist of more than one subunit. The bands visualized under reducing conditions reflect the size of the individual subunits.
nonreducing conditions: disulfide bonds remain intact. For proteins that consist of more than one subunit, the band will reflect the molecular weight of the entire protein.
What does Western Blotting do?
detects specific proteins with antibodies after an SDS Page
What does Southern blotting do?
used to detect specific DNA sequences within a complex DNA sample after gel electrophoresis. It can be used to analyze gene structure and organization and diagnosing genetic disorders.
What does Northern Blotting do?
used to detect specific RNA sequences within a complex RNA sample. It is very similar to Southern blotting for DNA. It can also be used to detect gene expression levels.
size exclusion chromotography
small molecules get trapped in the pores of the stationary phase, while large molecules flow through the gaps between the beads and have very small retention times. So larger molecules come out first.
pH > pI =
pH < pI =
the peptide will have a negative charge and migrate toward the positive electrode.
the peptide will have a positive charge and migrate toward the negative electrode.
gel electrophoresis + end name:
gel electrophoresis - end name:
anode, cathode
What are the 4 catalytic strategies enzymes use and what do they do?
acid/base: proton transer
covalent: electron transfer
electrostatic: stabilizing charge
proximity/orientation: increase succesfull collission rate
(also metal ions as cofactors to help stabilize intermediaries or activate things)
What are the two types of binding sites on enzymes and their differences?
allosteric, active
The active site is where the reaction happens and the allosteric site is where regulation via conformation change happens.
When are substrated most tightly binded to their enzymes in the induced fit model?
during the transition state
Whats a coenzyme?
subset of cofactors, organic, non-protein molecule that is required for the proper functioning of an enzyme. Coenzymes act as helper molecules, often facilitating the transfer of specific chemical groups, example is NADH where carries an electron or CoA, types of cofactors, seperate from the enzyme eventually
Whats a cofactor?
non-protein inorganic chemical compound or metal ion that is required for the biological activity of an enzyme, stabilize the transition state
vitamins and minerals generally refer to _ and _.
dietary cofactors and coenzymes
oxidoreductases
Catalyze redox reactions, where one molecule is oxidized (loses electrons) and another is reduced (gains electrons), can indirectly result in bond breakage or formation
transferases
Catalyze the transfer of a functional group (such as a methyl or phosphate group) from one molecule to another.
hydrolases
Catalyze the hydrolysis (breaking) of bonds by adding water, such as in the breakdown of larger molecules into smaller components, often an acid base reaction
lyases
Catalyze the addition or removal of groups (like CO₂, NH₃, or H₂O) to or from molecules, without hydrolysis or oxidation.
isomerases
Catalyze the rearrangement of atoms within a molecule to form an isomer, maintaining the same molecular formula.
ligases
Catalyze the joining of two molecules by forming a new covalent bond, often using energy from ATP (by rmoving a water) or another nucleoside triphosphate.
Types of Hydrolases
Proteases:
Lipases:
Nucleases:
Break down proteins into peptides and amino acids.
Break down fats into glycerol and fatty acids.
Break down nucleic acids into nucleotides.
High/low affinity proteins depending on concentration
transport proteins are generally more efficient at higher concentrations and may exhibit reduced efficiency at low concentrations due to lower affinity or less available substrate, example is hemoglobin for oxygen
A reaction is said to be _ _ if it happens quickly under the given conditions, i.e., it has a low activation energy.
kinetically favorable
A reaction that has to do with _ _ is talking about Delta G.
thermodynamic favorability
whats cooperativity in enzymes?
where increased substrate binding happens more quickly as substrate binding sites become occupied (positive), negative substrate cooperativity is when substrate binding decreases affinity for next substrates wanting to bind.
noncooperative substrate binding: where it is not affected
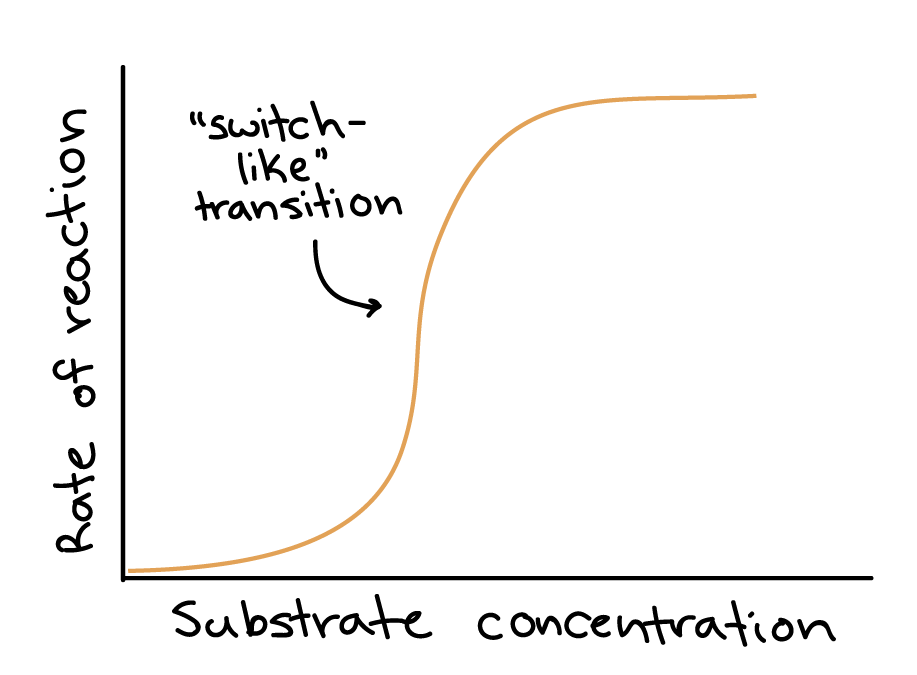
Good control points in feedback loops often have _ delta G’s.
very negative
Remember that not all enzymes are _.
proteins
whats a zymogen
is an inactive form of an enzyme that requires a biochemical change (usually proteolytic cleavage) to become an active enzyme.
Whats a noncompetitive inhibitor?
the inhibitor doesn't block the substrate from binding to the active site. Instead, it attaches at another site and blocks the enzyme from doing its job. This inhibition is said to be "noncompetitive" because the inhibitor and substrate can both be bound at the same time.
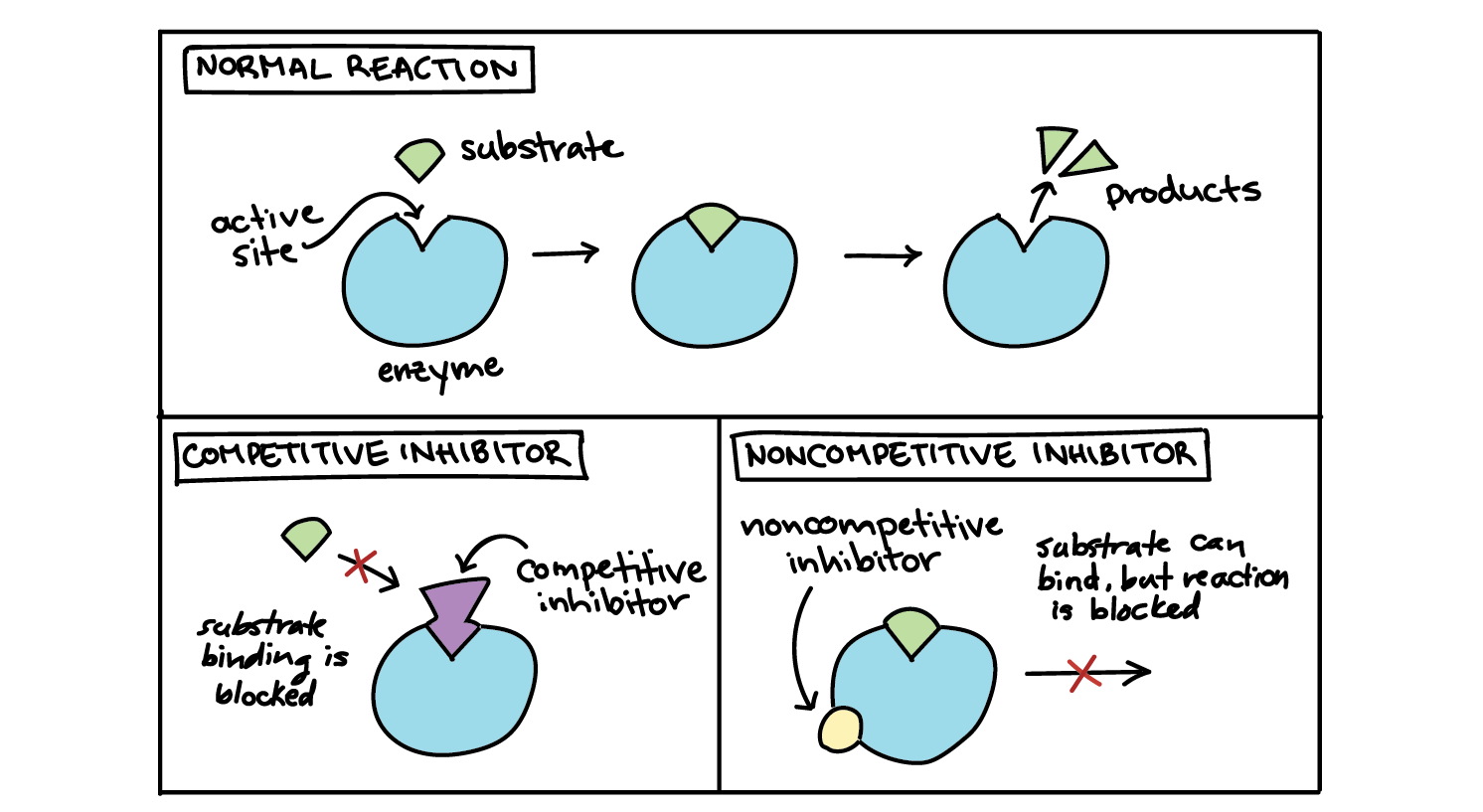
How does substrate concentration affect competitive inhibitors and noncompetitive inhibitors?
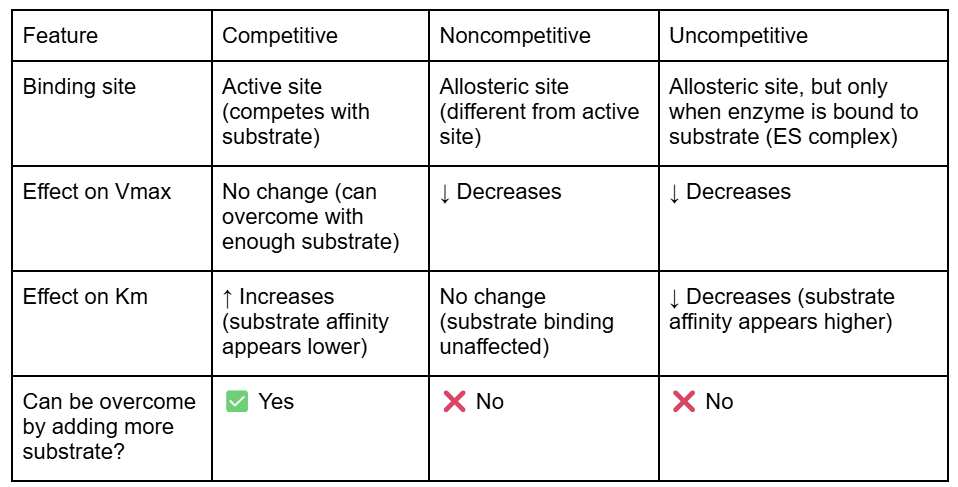
If an inhibitor is competitive, it will decrease reaction rate when there's not much substrate, but can be "out-competed" by lots of substrate. That is, the enzyme can still reach its maximum reaction rate given enough substrate. In that case, almost all the active sites of almost all the enzyme molecules will be occupied by the substrate rather than the inhibitor. With a competitive inhibitor, the reaction can eventually reach its normal \[V_{max}\], but it takes a higher concentration of substrate to get it there. In other words, \[V_{max}\] is unchanged, but the apparent \[K_m\] is higher.
If an inhibitor is noncompetitive, the enzyme-catalyzed reaction will never reach its normal maximum rate even with a lot of substrate. This is because the enzyme molecules with the noncompetitive inhibitor bound are "poisoned" and can't do their job, regardless of how much substrate is available. However, the reaction reaches half of its new \[V_{max}\] at the same substrate concentration, so \[K_m\] is unchanged. The unchanged \[K_m\] reflects that the inhibitor doesn't affect binding of enzyme to substrate, just lowers the concentration of usable enzyme.
![<p><span><span>If an inhibitor is competitive, it will decrease reaction rate when there's not much substrate, but can be "out-competed" by lots of substrate. That is, the enzyme can still reach its maximum reaction rate given enough substrate. In that case, almost all the active sites of almost all the enzyme molecules will be occupied by the substrate rather than the inhibitor. With a competitive inhibitor, the reaction can eventually reach its normal </span></span><span style="background-color: highlight; font-family: inherit; line-height: inherit; font-size: inherit;"><span>\[V_{max}\]</span></span><span><span>, but it takes a higher concentration of substrate to get it there. In other words, </span></span><span style="background-color: highlight; font-family: inherit; line-height: inherit; font-size: inherit;"><span>\[V_{max}\]</span></span><span><span> is unchanged, but the apparent </span></span><span style="background-color: highlight; font-family: inherit; line-height: inherit; font-size: inherit;"><span>\[K_m\]</span></span><span><span> is higher.</span></span></p><p>If an inhibitor is noncompetitive, the enzyme-catalyzed reaction will never reach its normal maximum rate even with a lot of substrate. This is because the enzyme molecules with the noncompetitive inhibitor bound are "poisoned" and can't do their job, regardless of how much substrate is available. <span><span>However, the reaction reaches half of its new </span></span><span style="background-color: highlight; font-family: inherit; line-height: inherit; font-size: inherit;"><span>\[V_{max}\]</span></span><span><span> at the same substrate concentration, so </span></span><span style="background-color: highlight; font-family: inherit; line-height: inherit; font-size: inherit;"><span>\[K_m\]</span></span><span><span> is unchanged. The unchanged </span></span><span style="background-color: highlight; font-family: inherit; line-height: inherit; font-size: inherit;"><span>\[K_m\]</span></span><span><span> reflects that the inhibitor doesn't affect binding of enzyme to substrate, just lowers the concentration of usable enzyme.</span></span></p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/ccd85ccc-1728-4961-bf84-fd4601406a75.png)
uncompetitive inhibitors
bind exclusively to the enzyme-substrate complex, not to the free enzyme. This binding typically occurs at a site distinct from the active site, forming an enzyme-substrate-inhibitor complex that is catalytically inactive.
Hill coefficient interpretation

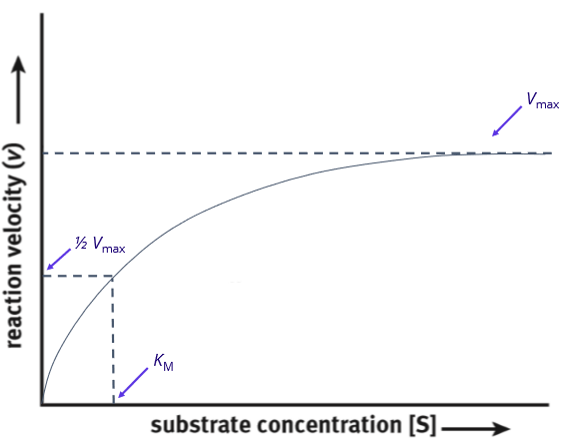
Michaelis menton plot
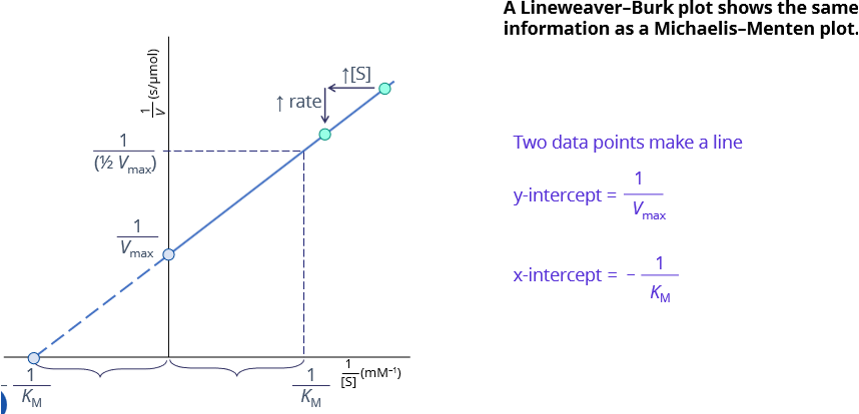
lineweaker burke plot
How the 3 inhibitor types are affected by substrate concentrations
how do enzymes catalyze forward and reverse reactions?
the same enzyme does both equally and just speeds up how fast equilibrium is reached, with the exception being high -G reactions that have crazy activation energies
keto-enol tautomerization reaction
where electrons/protons are distributed in bonds within the same molecule
holoenzyme
the complex of an enzyme that has its cofactor bound and is considered ‘complete’.