Ion channels L4
1/80
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
81 Terms
Classify ion channels based on their structure and gating mechanism
open/leakage channels - 4 subunits, 2 helices, controlled by structure of channel
voltage gated - 4 subunits, 6 to 24 helices, p loop, cytoplasmic anchors, voltage sensing domains, plugging mechanism
ligand gated, both IC and EC - 4 subunits, 6 helices, cytoplasmic anchors
Explain plugging mechanism in voltage-gated ion channels
intracellular portion of the channel acting as a "plug" or "ball and chain", blocks the pore shortly after it opens. rapid closure, even when the membrane is still depolarized, stops ion flow and is crucial for the channel's function and the generation of action potentials by creating a refractory period
Identify features and function of ligand-gated ion channels.
excitability, signalling, secretion, absorption, and muscle function
Explain the structural basis of ion selectivity and describe how pore architecture and amino acids determine which ions pass through
pore architecture: selectivity filter, narrow region of the pore that matches the size and charge of the target ion, only ions that can fit and shed their hydration shell correctly can pass
amino acids: Carbonyl oxygens or charged residues line the filter, their spacing mimics the ion’s normal hydration envt, stabilising the right ion but not others
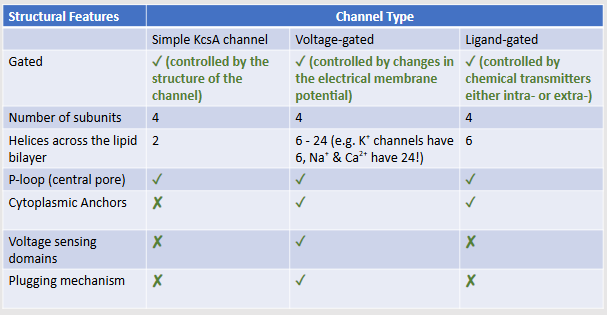
Compare and contrast key features of different ion channels
Describe how dysfunction of ion channels can contribute to disease.
Mutations or misregulation of ion channels contribute to conditions such as epilepsy, stroke, ALS, glioblastoma, and nicotine addiction.
Evaluate how ion channels serve as drug targets
Ion channels are attractive drug targets because of their diversity and specificity, physiological importance, and role in disease.
What is a transmembrane protein?
protein channel that transports molecules from one side of the membrane to the other
What are ion channels specific to?
open or gated, specific to sodium, potassium, chloride
What are essential functions carried out by ion channels?
transport ions across membrane (secretion/absorption of fluids)
regulate membrane potentials (nerve + muscle cells for high-speed communication)
calcium influx into the cytoplasm (secretion + muscle contraction)
Name examples of areas where ion channels transport fluids/ions across membranes
salivary glands and kidneys
Name a way in which ion channels can regulate membrane potentials. Where would these channels therefore most likely be found and why?
open channels = ions to diffuse down the concentration gradients into the cell so can control the membrane potential = electrical signal that spreads very rapidly over the cell surface
nerve and muscles cells use these action potentials for high speed communication
Name a way in which ion channels allow secretion and muscle contraction
Ca2+ influx into the cytoplasm from ER or outside the cell where it can be used for a number of processes such as secretion or muscle contraction.
What are common structural features to all ion channels?
transmembrane, two or more alpha helices crossing lipid bilayer
2 - 6 subunits surrounding a pore
What structural differences are ion channel subgroups based on?
Gating mechanism
Ion Selectivity of the pore
What defines the ion selectivity of a pore?
by physical size of ‘filter’ and amino acids lining the pore
How many genes in humans code for membrane channels?
400
Considering that structure of ion channels has revealed evolutionary relationships between ion channels, what can the KscA potassium channel found in bacterium be used for?
serves as a model for all channels
Describe the molecular structure of a simple potassium ion channel like the KcsA channel
highly selective TM helicase structure forming a p - loop (pore)
on cytoplasmic side, TMs more tightly packed = gate
What 3 factors control a gate on a simple ion like potassium’s channel?
membrane potential
mechanical stress
ligands
What are the 2 main functions of voltage gated ion channels?
sodium and potassium create action potentials in excitable cells
calcium is transported into cytoplasm where 2nd messenger = cellular response
What distinguishes a voltage gated ion channel from a simple ion channel?
Additional helices S1 and S4 form a separate ‘voltage sensing domain’ lateral to the subunits
Large polypeptides that extend into the cytoplasm
Plugging mechanism

Explain how this voltage gated potassium ion channel opens/closes
Voltage-gated K⁺ channels have 4 subunits, each with 6 transmembrane helices (S1–S6).
S4 helix, rich in positively charged residues, senses changes in membrane potential.
Depolarisation → S4 moves outward → S4–S5 linker pulls open S6 → channel opens.
Repolarisation → S4 returns inward → S6 closes → channel closes.
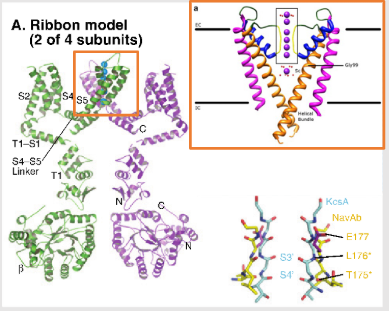
Describe the ion channel seen on this image
Voltage gated potassium ion channel:
The ribbon model shows two of four subunits of a Kv channel.
Each subunit has six transmembrane helices (S1–S6).
S1–S4 make up the voltage-sensing domain (VSD).
S5–S6 form the pore domain, including the selectivity filter and activation gate.
The S4 helix (positively charged) is key to sensing voltage changes.
Describe the resting state of a voltage gated potassium ion channel
The inside of the cell is negatively charged relative to the outside.
The positively charged S4 segment is attracted inward (toward the cytoplasm).
This keeps the activation gate (at the S6 helices) closed, preventing K⁺ flow.
Describe a voltage gated potassium ion channel when depolarising/activating
membrane potential becomes less negative:
The reduction in negative potential repels the S4 segment’s positive charges, causing it to move outward (toward the extracellular side).
This movement pulls on the S4–S5 linker, which in turn opens the activation gate in the S6 region → K⁺ ions flow out.
Describe a voltage gated potassium ion channel when repolarising/inactivating
When the membrane potential returns to negative, S4 moves back inward, releasing tension on S4–S5.
This allows the S6 helices to close again, blocking the pore.
Some Kv channels also have a “ball-and-chain” inactivation mechanism, where a polypeptide segment (often from the N-terminus, not S4) transiently plugs the pore.
How are ligand-gated ion channels similar and different from voltage-gated ion channels?
similar structure, just controlled by different factors, former by the binding of a ligand
Are ligand-gated channels controlled by IC or EC ligands? Name examples of these types of channels?
can be either ! IC is eg Cyclic nucleotide ligand, Ec is eg nicotine, glutamate, ATP
How many subunits and helices go across the lipid bilayer in simple KcsA, voltage-gated and ligand-gated channels?
4 subunits for all, 2 helices for KcsA , 6 to 24 for voltage-gated and 6 for ligand-gated
Which types of ion channels have a p loop, a cytoplasmic anchor, a voltage sensing domain/a plugging mechanism?
voltage-gated has all, ligand-gated has p loop and cytoplasmic anchors and KcsA just has the p-loop
What cellular process are extracellular ligand-gated ion channels important in?
cell-cell communication
What are distinct families of extracellular ligand-gated receptors? Are they tri/tetra/penta - metric?
ATP 2X : trimeric P2X 1 to 7
glutamate: tetrameric AMPA, NMDA, KA
nicotinic receptor superfamily: Pentameric nAChRs, 5-HTR, GABAaRs, GlyRs, GluCl, MOD-1
Name some specific functions of channels selective for sodium or potassium
control membrane excitability – depolarize cells
Name some specific functions of channels with added permeability to calcium
directly regulate activity of calcium sensitive proteins
Name some specific functions of channels that are chloride selective
control membrane excitability – reduce resistance/ hyperpolarize cells, reduce action potential firing
In the specific example of a cys-loop type receptor like the nAChR, describe its structure in muscle
5 subunits (alpha, beta, gamma, epsilon)
each subunit has 4 TM M1, M2, M3, M4 with
a large external facing N domain and IC loop between M3 and M4.
M2 lines the pore.
have small IC domains anchoring them into the cell membrane
In the specific example of a cys-loop type receptor like the nAChR, describe its mode of action towards muscle contraction
NT binds - opens channel - non selective flux of cations - electrical change = muscle contraction
How is added complexity added into ligand-gated ion channel families? How does this affect drug targeting?
different subunit combinations make up receptors in different parts of the brain - complexity = diversity ie opportunity for specific drug targeting
Which ligand-gated ion channel is involved in reward pathways and nicotine addiction? How could it differ from other channels within the same ligand-gated ion channel family?
nACh alpha4 - different subunits to others within same family like nAChalpha 1, 2, 3, 7, 9, 10 etc
Which subunit variations define nAChR affintiy for nicotine? What else could affect this?
alpha 2 to 10 and beta 2 to 4 - huge amount of variability between these receptors depending on subunit associations
composition and location of receptors and subunits
Which type of receptor is abundantly expressed in the cortex and hippocampus and have high affinity to agonists nicotine and varenicline?
nACh alpha 4 beta 2
What does chronic exposure of nicotine receptors to agonist lead to?
receptor upregulation ie more nicotine = more receptors made = more addiction
When conducting genetic studies on nAChRs, what polymorphisms in subunit genes were found to be linked to tobacco dependence? What does this knowledge allow?
What other variants have been found that could allow for treatment against tobacco dependence?
subunit genes CHRNA$ (alpha 4) and CHRNA6 (alpha 6) - linked to tobacco dependence
variants shown to be protective against dependence - could be exploited for treatment?
What causes autosomal dominant nocturnal frontal lobe epilepsy? How many mutations have been identified causing ADNFLE?
mutation in the M2 region of alpha 4 neuronal nicotinic subunit so in nAChR
9!
How do mutations in M2 region of alpha 4 neuronal nicotinic subunit affect nAChR function and cause ADNFLE?
M2 mutations in α4 subunit alter the ion pore → increase receptor open probability and slow desensitization → enhance nicotinic signalling → cortical hyperexcitability → ADNFLE seizures.
How does a sustained depolarised nACh M2 mutated receptor lead to ADNFLE?
Mutant α4β2 nAChRs in thalamocortical neurons become overactive.
excessive ACh-induced depolarisation and enhanced transmitter release.
The neuronal network becomes hyperexcitable, especially during non-REM sleep, when ACh levels fluctuate.
This leads to seizure activity localized to the frontal lobe, producing nocturnal motor seizures.
Which is the main NT in the brain? Briefly describe the structure of the receptor to which this NT binds
glutamate
glutamate receptors are tetramers similar to KcsA but the pore is inverted
form as dimer of dimers, ligand binding site is in a cleft that closes when occupied
What contributes to glutamate receptor diversity? Name examples of these receptors
Give some examples of glutamate Kainate receptors
GluK 1 - 5
Name some examples of glutamate NMDA receptors
GluN1, GluN2 A- D
Name examples of glutamate AMPA receptors
GluA1 - 4
What is the role of AMPA receptors?
mediate fast excitatory synaptic transmission in the central nervous system
What is the role of NMDA receptors?
N-methyl-D-aspartate receptor – involved in learning and memory – slower than other isoforms
What is the role of kainate receptors?
similar to AMPA but lesser role at synapses linked to Schizophrenia, depression and Huntingtons
What are 2 types of RNA processing that can affect receptor subunits?
RNA splicing and editing
How do the kinetic properties of flip differ from those of flop in AMPA receptors?
flop is desensitised at a quicker rate and has a reduced current response to glutamate than flip
How does the presence of isoforms of AMPA receptors throughout different regions of the brain allow for tuning of synaptic responses and plasticity?
Different brain regions express different ratios of flip/flop isoforms = tuned synaptic responses and plasticity ie how long AMPA receptors stay active after glutamate release
What are the two isoforms under which each subunit of AMPA receptors exist?
2 splicing isoforms of the subunit genes like GluA 1-4 - flip and flop, basically EC ligand binding loop of the proteins is different due to alternative splicing of 2 exons in the primary transcript
In an AMPA receptor like GluA2, where is the Q/R site located and what is its function?
located in the M2 of the subunit, inside the channel pore
RNA editing site
How does the Q/R site of an AMPA receptor carry out RNA editing?
RNA editing enzyme ADAR2 converts the genomically encoded CAG (glutamine, Q) into a CGG at the RNA level = CGG (arginine, R) in the protein ie a Q to R site where rna is edited from original dna code
How do functions of unedited Q RNA differ from edited R RNA in AMPA receptors?
unedited allows calcium through the AMPA receptor so has a high permeability to it // edited blocks calcium entry so has a low calcium permeability
What does the edited R form of AMPA receptors protect neurons from, considering their permeability to calcium? Are there more edited or unedited GluA2 subunits in the brain’s AMPA receptors?
excitotoxicity
more edited ie R form - makes AMPA receptors largely impermeable to calcium, maintaining neuronal stability
What happens in cases of failure for RNA to be edited at the Q/A site of an AMPA receptor? What could cause this type of failure?
AMPA receptors would be calcium permeable in their Q form = too much calcium = neuronal hyperexcitability, seizures and cell death
loss of ADAR2 enzyme or ADAR2 knock-out
Considering the speed of desensitisation of flip isoform AMPA receptors, what type of synapses are these receptors mostly involved in? How fast is their recovery time and what type of response do they have to glutamate?
slow desensitisation so involved in synaptic plasticity allowing learning and memory - fast recovery and sustained response to glutamate
Considering the speed of desensitisation of flop isoform AMPA receptors, what type of synapses are these receptors mostly involved in? How fast is their recovery time and what type of response do they have to glutamate?
fast desensitisation so involved in rapid synapses like high-frequency transmission and precise timing often found in auditory circuits
slow recovery and short responses to glutamate
Where would there be a higher flip to flop ratio of AMPA receptors in the brain?
hippocampus
Where would there be a higher flop to flip ratio of AMPA receptors in the brain?
cerebellum and auditory neurons
What could a pathologically high expression of flip AMPA receptors lead to?
longer activation and slower desensitisation = Hyperexcitability, risk of seizures or excitotoxicity
What could a pathologically high expression of flop AMPA receptors lead to?
rapid desensitisation and reduced glutamate response = synaptic weakening, potential cognitive deficits
Consequence of AMPA dysfunction
calcium permeability of AMPA = seizures and death
Consequence of NMDA dysfunction
stroke and neuron death
What is thought to be the role of NMDA receptors?
controlling synaptic plasticity and mediating learning and memory functions
In glioblastoma, decreased ADAR2 activity correlated with increased malignancy bc of an increase in Ca2+ = Akt pathway promoting proliferation and tumorigenesis. What is therefore a potential therapeutic application?
GluA2 Q/R was editing = increasing survival bc increased nb of edited AMPA receptors than unedited reduced the calcium and the Akt pathway, reducing proliferation of tumours
Name some pathological conditions in which glutamate receptor RNA editing is involved
forebrain ischemia, amiotrophic lateral sclerosis (both GluA2 receptor subunit), spinal cord injury (GluA2, 3, 4 and GluK1, 2), excitotoxicity, epilepsy, schizophrenia, bipolar disorders, antidepressant treatments, glioblastoma, fear conditioningm al
What type of ligand binds to P2X receptors? Briefly describe its structure
adenosine triphosphate (ATP)
trimeric assembly, 3 subunits with 2 TM helices, large EC domain
How many molecules are needed to open a P2X channel?
3 ATP molecules needed to open channel
What types of subtypes of subunits do P2X receptors have?
P2X 1 to 7
Consequence of P2XR dysfunction
microglia: modified stimulation of BDNF synthesis in microglia - depolarising shift in the anion reversal potential underlying neuropathic pain
macrophages: modified activation of the caspase 1 inflammasome, release of inflammatory cytokines
neurons: activation in stroke induces excitotoxic neuronal cell death by calcium overload. P2X 7 blockade = reduced brain tissue damage after tMCAO
Define gating
conformational mechanism by which ion channels open or close in response to voltage, ligand binding, or mechanical force. It allows neurons to control ion flow precisely, enabling electrical signalling, synaptic transmission, and sensory responses.
What causes ion channel diversity?
multiple subunit isoforms, RNA editing, alternative splicing