Chapter 1-3 Summer Work
1/56
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
57 Terms
Matter
Solid has a fixed shape and volume
Liquid has a fixed volume but takes the shape of the container
Gas has neither fixed volume nor shape
Pure substances
Element or compound
Element
Cannot be broken down into two or more pure substances ( it’s already in it’s purest form)
Compounds
A pure substance that contains more than one element
Fixed composition ( always contains the same elements in the same mass percentages —> ex water always has 2 hydrogen molecules and 1 oxygen molecule)
Properties of compounds are different than its elements that make it up —> ex: Sodium Chloride is unreactive but sodium is extremely reactive and chlorine is poisonous
techniques such as electrolysis and heat are used to separate the compound into its pure substance
Uniform properties and lack of physical mixing with other substances
Mixtures
Contain two or more substances that retains chemical identity ( physically combined but not chemically) so they contain their original chemical properties
Homogenous Mixtures
When the composition of the mixture is the same throughout. Another name is a solution ( a solvent and a solute)
Examples: salt water, vinegar, coffee without milk, soft drinks
Heterogenous mixtures
When the composition of the mixture varies throughout such as rocks ( can contain different % of mass of elements/ substances)
Differing components/ non– uniform
How to separate components of a mixture: Filtration
Filtration separates a HETEROGENEOUS solid – liquid or solid– gas mixtures. It uses filer paper where the liquid part goes through and the solid stays in the filter paper.
How to separate components of a mixture: Distillation
Distillation separates HOMOGENOUS solid–liquid or liquid– liquid mixture. The liquid vaporizes, leaving a residue of solid in the distilling flask ( uses heat)
Heats the mixture to vaporize the more volatile ( lower boiling point) which travels to a condenser. Then it is cooled down back to a liquid.
How to separate components of a mixture: Chromatography
Chromatography separates HOMOGENOUS Gas liquid or solid liquid mixtures
Gas Chromatography vaporizes a liquid or solid as it travels through the column
Compounds with lower boiling point move through the column faster, resulting in a chromatogram, a graph that plots intensity versus time.
Units of Measurement and instruments
Standard Unit of length of is Meter.
Volume is measure in cubic centimeters, liters, and milliliters.
mass is expressed in grams, kilograms, or milligrams.
Mass
A measure of the amount of matter in an object
Weight
A measure of the gravitational force acting on the object
Temperature
determines the direction of heat flow
Heat flows higher temp to lower temp
SI Unit is kelvin
Significant Figures
Used when there is uncertainty of the measurements when measuring volume
Meaningful digit obtained in a measurement
RULES for Sig Figs: —> Non– Zeros, Zeros between digits, and zeros after the digit with decimal points are significant ( zeros in front of digits aren’t)
Rules for adding and subtracting, and multiplying and division
For multiplying and dividing the number of sig figs is the same as the smallest number of sig figs in a number
For adding and subtracting the number of decimal places which is the smallest is the amount of sig figs you should have ( ex .355 + .8976 would lead the total to have 3 sig figsa
Rules for rounding off
If the digit is to be discarded is less than 500 leave it alone
if the digit to be discarded is more than 500 round up
If the digit to be discarded is = 500 round so its an even number
Conversion of units
1m=100 cm
1m=1000mm
1m=1 × 10^9 nm
1 ft =0.305 m 12 in
1 yd =0.914 m 3 ft
1 mi=5280 ft
1 in =2.54 cm
1 cm³= 1 ml, 1 l =1.0567 qt, 1 ft³= 28.32 L
1 kg=1000 g
1 g=1000 mg
1 lb= 16 oz
1 lb=453.6g
Properties of substances:
Intensive: Must be independent ( separate) of the amount of substance and doesn’t change even different mass such as density, boiling point, etc.
Extensive: Properties that depend on the amount of substance such as mass and volume
Chemical Properties:
Properties that occur when the substance takes part in a chemical reaction where it is changed to a new substance.
Examples are: Flammability, toxicity, reactivity with other substances, heat of combustion, and the ability to break down other substances ( Corrosivity)
Physical Properties:
Properties without changing the chemical identity of a substance.
Examples are: Melting point —> the temperature at which substances change from liquid to solid ( Substances don’t change identity when melted just their states change)
Boiling point —→ the temperature at which vapors ( a gas forms)
Density
Units of conversion
1 Kilometer =1000 meters
1 meter= 10 decimeters
1 meter=100 centimeters
1 meter=1000 milimeters
1 meter=10^6 micrometers (u)
1 meter=10^9 nanometers (n)
Atoms:
An Element composed of tiny particles. All atoms of a given element, have the same chemical properties, while atoms of different elements have different properties.
Dalton Theory
The Atom can be defined as the smallest particle of an element that can enter a chemical reaction
He believed All atoms of a given element are identical in mass and properties
In ordinary chemical reactions atoms move from substance to substance but no atom of any element disappears or is changed into another atom.
In a compound the relative number of atoms are integers or simple fraction.
Different combinations produce different compounds
Atoms of different elements have different masses
Components of the atom
JJ Thomson discovered the Electron in which particles passed through the cathode ray tube. The rays or particles deflected from the positive pole showing that it is negatively charged.
Electrons carry a -1 charge and weight 1/2000.
The Law of conservation of mass
John Dalton discovered that mass can’t be created or destroyed.
Law of constant composition
Compounds always contain the same elements in the same proportions by mass, regardless of how prepared.
Law of multiple proportions
John Dalton discovered that Two elements combine to form different compounds, the ratios of the masses of one element to another are whole numbers
Protons and Neutrons
JJ Thomson suggested the plum pudding model where there is a sphere of positive charge and negatively charged electrons embedded like plums on top of pudding.
Rutherford did an experiment where he passed/ fired a particles ( helium atoms without their electrons) and most went through the foil without changing their direction but a few reflected back at acute angles.
Scattering was caused by a positively charged nucleus at the center of the foil’s atom. Most of the a atoms is empty space which is why the bouncing occurred vs passed through
The proton has a mass of nearly 1 carries a +1 charge. The neutron an uncharged particle with mass also a bit more than 1.
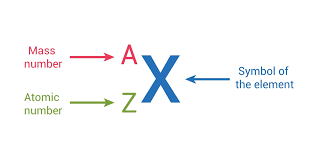
Atomic number
All atoms of a particular element have the same number of protons —> symbol z = # of protons
in a neutral atom the # of protons equals the # of electrons
Atomic masses
How heavy atoms of different elements are
Mass numbers; isotopes
Mass number is represented by A= # of protons + # of neutrons
All atoms of a particular element may differ in mass because the # of neutrons can be different
Istopes: Atoms with the same # of protons but different # of neutrons
To Find the average atomic mass you do mass number of the element ( times the percentage of abundance/100) + the other elements.
Isotopic Abundances:
The parentage of a specific isotope within a naturally occurring element —> total adds up to 100% —> used to calculate the element’s average atomic mass.
By comparing the accelerating voltages required to bring ions to the same point on the detector, it is possible to determine relative masses of ions.
Mass Spectrometry:
The atomic masses ( relative abundance) of the two isotopes are determined by the proportions of the heights of the peaks —> the x– axis determine that the isotopes % have the quantity of mass ex) Cl —> 75% have a mass of 35 amu
Masses of individual atoms; avogardo’s number
Na= 6.022 × 1023 ( represents the number of atoms of an element in a sample whose mass in grams is numerically equal to the atomic mass of the element)
Periods and Groups
The horizontal rows are periods ( ex: Li-Ne)
The vertical column, 1 - 18 are groups
Groups 3–12 are transition metals
Groups 1,2,13,14,15,16,17,18 are main group elements
Group 1 is alkali metals
Group 2 us alkaline earth metals
Group 17 is Halogens
Group 18 is noble gases
*The periodic table is an arrangement of elements, in order of increasing atomic number. Elements in vertical groups have similar chemical properties.
Dimitri Mendeleev invented the periodic table
Metals and nonmetals
Groups 1 – 16, except H, B, Is, Ge are metals
Metals have high electrical conductivities
Boron, Silicon, Ge, Arsenic, Sb, Te are metalloids because their properties fall between
Groups 14– 18 are non– metals
Molecules
Two or more atoms combine to form an uncharged molecules
Within the two atoms ( commonly as non– metallic forces) called covalent bonds are sharing electrons —> intermolecular forces ( the ones that hold separate molecules together are quite weak)
Structural formulas
Shows the bonding pattern within the molecule
Condensed Structural formula
Suggests the bonding pattern in the molecule and highlights
the presence if a reactive group of atoms within the molecule
Ions
When an atom loses or gains electrons, giving it a net charge
Metal atoms lose electrons and form cations
Non metals gain electrons and form anions
The atomic number doesn’t change when an ion is formed
Polyatomic ions are held by covalent bonds
Ionic bonds
Strong electrical forces holding oppositely charged ions ( a cation and an anion)
Ionic compounds are solids and have high relatively melting points.
To melt ionic compounds they must be separated —> When dissolved they conduct an electric current making it a stronger electrolyte
Discrete molecules
Separate units, held be strong covalent bonds, neutral
attracted to each other by intermolecular forces
Aren’t solids, rather move around
Ocet rule
Atoms that are close to a noble gas in the periodic table form ions that contain the same number of electrons as the neighboring noble gas atom to try to form a stable electron configuration.
transition and post– transition metals form positive ions and more than one.
Polyatomic ions positive
Ammonium ( NH4+)
Hydronium ( H3O+)
Polyatomic -1
Acetate ( C2H3O2-1)
Hydroxide (OH-1)
Nitrate ( NO3-1)
Chlorate ( ClO3-1)
Nitrie ( NO2-1)
polyatomic -2
Carbonate ( CO3-2)
Sulfate(SO4-2)
polyatomic -3
Phosphate (PO4-3)
Binary Molecular Compound
Product of when two nonmetals combine with each other
Uses the prefixes di,tri,tetra,penta,hexa,hepta,octave,nona,and deca
The second half ends it wide
Naming ionic compounds
When a nonmetal forms two oxanions ate is used for the anion with the larger number and its is used for the anion containing few oxygen atoms.
Naming acids
If name of anion ends in ide then the acid name is hydro___ ic —> Ex)HCL —> hydrochloric acid
If name ends in ite(polyatomic) than acid name is ___ ous
—> EX) HNO2 —> nitrous acid
If name ends in ate than acid name is ____ic
—→ EX) H2SO3 —→ Sulfurous acid
Acids are substances that ionize ( gain or lose an electron) in water to produce hydrogen ions (H+)
Percent composition
Found by the mass of the element present and the mass of the substance
—> EX) 11.19 g H/100 = 11.19% H
When given mass percents in a compound assume 100 grams
Combustion
Mass of sample= mass of O+ mass of C + mass of h
OR mass of O= mass of sample - ( mass of C + mass of H)
Theoretical yield
The maximum amount of product that can be formed in a chemical reaction
Found by using the limiting reactant and whatever you get as the total for the product is theoretical yield
Percent yield
actual yield ( experimental)/theoritcal x 100
How to find excess reactant’s mass
Can find it by subtracting the amount of moles in the product to the amount of moles of the excess reactant and converting
OR by using the mass of the limiting reactant converting to moles of the excess and multiplying by the molar mass to find the amount of mass used up. Subtract that by the mass given in the problem.
Mass Spectra of diatomic elements
Elements that are diatomic such as Chlorine, oxygen, nitrogen, etc. may have more lines on their mass spectra because their atoms may stick together