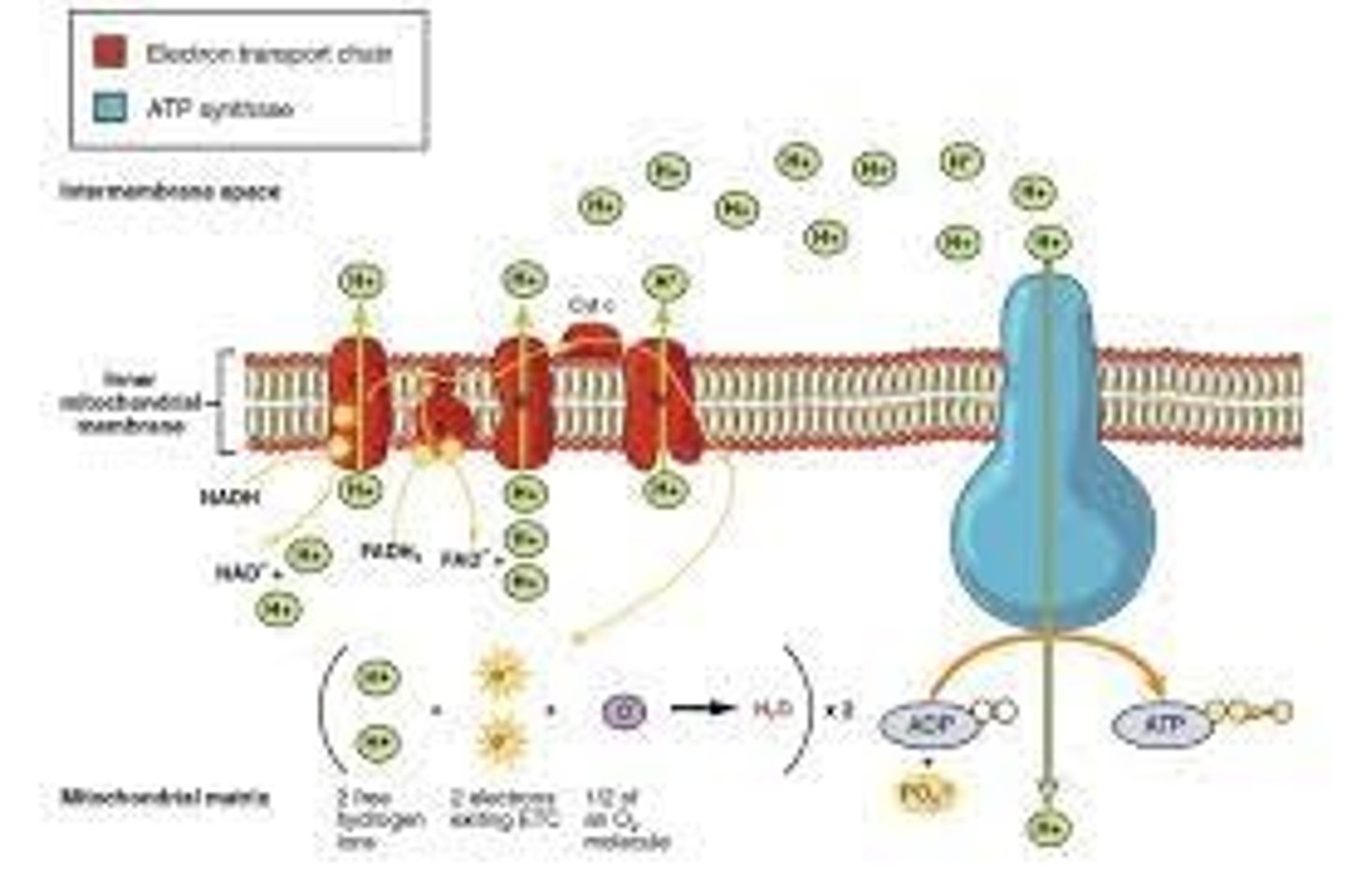
Detailed Study Guide on the Electron Transport Chain for Biology Course
1/82
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
83 Terms
The electron transport chain will pass electrons from NADH/FADH2 to ___
oxygen
How many electrons does it take to fully reduce a molecule of oxygen?
4 electrons (2 pairs)
What is the reaction for the reduction of oxygen?
2e- + 1/2 O2 + 2H+ --> H2O
NADH and FADH2 are reoxidized by:
carriers in the electron transport chain
Where are the electron transport chain proteins embedded?
inner mitochondrial membrane
The inner mitochondrial membrane is folded into structures called:
cristae
The inner mitochondrial membrane is highly _____ to most outside particles
impermeable
70% of the inner mitochondrial membrane is composed of ______, while 30% is composed of _____
protein; lipid
The outer/inner mitochondrial membrane is separated by the ___ ___
intermembrane space
What is the fluid-filled space INSIDE the inner mitochondrial membrane?
mitochondrial matrix
Where does the citrate cycle take place?
mitochondrial matrix
The operation of the electron transport chain will create an ____ ____
electrochemical gradient
NADH and FADH2 are found within the
mitochondrial matrix
When NADH is oxidized to NAD+, H+ is pumped from the _______ to the ______
matrix --> intermembrane space
When H+ is pumped from the matrix to the intermembrane space, there is a large potential energy within the intermembrane space, known as:
proton-motive force
When protons flow down their gradient into the matrix, they must pass through
ATP synthase
When protons flow down their gradient into the matrix, the negative ΔG reaction will power:
ATP synthesis
The oxidation of NADH/FADH2 will phosphorylate ADP to produce ATP.
This reaction is known as
oxidative phosphorylation
The transfer of electrons between carriers in the electron transport chain are all -ΔG reactions.
These reactions will drive:
the pumping of H+ against their gradient into the intermembrane space
The electron transport chain is also known as the ____ ___
respiratory chain
The electron transport chain is composed of _____ large multiprotein complexes
4
The 4 large multiprotein complexes of the ETC are embedded within the
inner mitochondrial membrane
In addition to the four large protein complexes. there are 2 ___ ____ that shuttle between complexes
mobile carriers
What are the electron carriers in the ETC?
Coenzyme Q and Cytochrome C
What electron carrier shuttles from Complex I/Complex II to Complex III?
Conenzyme Q
What electron carrier shuttles from Complex III to Complex IV?
Cytochrome C
Each of the components in the ETC has increasing ___ ___
reduction potential
What are the two flavoproteins in the ETC?
1. Flavin mononucleotide (FMN)
2. Flavin Adenine Dinucleotide (FAD)
FAD will enter the electron transport chain in: (FAD--->FAD2H)
Complex II
NADH will enter the electron transport chain in: (NADH ---> NAD+)
Complex I
Coenzyme Q is also called:
ubiquinone
Coenzyme Q is _____, and as a result, can diffuse within the inner mitochondrial membrane
hydrophobic
Cytochrome C is a soluble carrier that is associated with the ______ _____ of the inner mitochondrial membrane
outer surface
The energy generated during the transfer of electrons isn't used to DIRECTLY synthesize ______ , but is used to pump protons against their gradient
ATP
The driving force for protons to go into the mitochondrial matrix is known as:
proton-motive force
Complex I (NADH-Q Oxidoreductase) catalyzes:
1. oxidation of NADH
2. reduction of coenzyme Q
Complex II (Succinate-Q reductase) catalyzes:
1. oxidation of succinate
2. reduction of Coenzyme Q
Complex III (Q-cytochrome C oxidoreductase) catalyzes:
1. oxidation of coenzyme Q
2. reduction of cytochrome C
Complex IV (cytochrome C oxidase) catalyzes:
1. oxidation of cytochrome C
2. reduction of oxidase
Which complexes transfer protons into the intermembrane space?
Complex I, III, IV
Which complexes DO NOT transfer protons into the intermembrane space?
Complex II
After NADH is reduced, the electrons are passed from Complex I to _____
Complex III
How many protons does Complex I pump into the intermembrane space?
4H+
How many protons does Complex III pump into the intermembrane space?
2H+
How many protons does Complex IV pump into the intermembrane space?
2H+
Inner mitochondrial membranes are impermeable to _____, so there must be a "shuttle" that will bring its electrons into the matrix
NADH
To get electrons from NADH into the matrix, muscle cells use
glycerol-3-phosphate (G3-P) shuttle
The electrons on FADH2 are picked up by:
Coenzyme Q
In liver cells, electrons will be transferred from NADH onto _____ via ____
oxaloacetate; malate dehydrogenase
When oxaloacetate receives electrons from NADH, it will now be converted into
malate
When malate is transported into the matrix, it will enter the ___ ___, and will generate ____
TCA cycle; NADH
In the liver, electrons from NADH will enter the electron transport chain at
Complex I
Why does FADH2 yield less ATP than NADH?
FADH2 electrons enter the electron transport chain at Complex II, which does NOT pump protons into the intermembrane space
ATP synthase is also known as
F1/F0-ATPase
The F1 component of ATP synthase is found in the ____ ____
mitochondrial matrix
The F0 component of ATP synthase is found in the
inner mitochondrial membrane
The F1 component of ATP synthase (matrix component) contains ____ _____ activity
ATP synthesizing
The F0 component is largely _____, and is ______
hydrophobic; membrane-spanning
The F1 subunit is considered the _____ channel
catalytic
The F0 subunit is considered the _____ channel
proton-conducting
Each beta subunit in the hexameric ring (F1) will interact with a distinct surface of the ____ ____
gamma stalk
What determines which conformation each beta subunit will be in?
its interaction with the gamma stalk
What drives the rotation of the gamma stalk?
the transport of H+ from the intermembrane space into the matrix
The synthesis of 1 ATP molecule requires how many protons to be transferred into the matrix via ATP synthase?
3 H+
IN TOTAL, how many protons are required to synthesize 1 ATP molecule (ATP synthase AND Pi translocase)?
4 H+
Per NADH, how many protons are pumped into the intermembrane space?
10 H+
Per FADH2, how many protons are pumped into the intermembrane space?
6 H+
6/4 = 1.5 ATP per FADH2
Under aerobic conditions, how much ATP is generated per glucose molecule?
30 ATP
ATP synthesis is ____ ____ to the inward flow of H+
tightly coupled
1. ATP is not synthesizes unless H+ flows into the matrix
2. H+ does not flow into the matrix unless ATP is being synthesized
Under low activity levels, NAD+ is produced in LOW LEVELS and the citrate cycle will be ______
inhibited
The outer mitochondrial membrane is _____ to most small molecules via porins
permeable; non-selective
The inner mitochondrial membrane is _____ to most small molecules
impermeable
[Complex 1] NADH Ubiquinone Oxidoreductase
contain prostatic groups: (they help passing e⁻)
1. FMN - Flavin mononucleotide
2. Fe-S clusters (Iron-sulfur clusters)
This complex catalyzes:
1. transfer of e⁻ from NADH -> FMN -> Fe-S clusters -> UQ**
** ubiquinone
2. pump 4H⁺ ions, from the matrix to intermembrane space.
net:
NADH+H⁺+UQ+4H⁺(in Matrix) --> NAD⁺+UQH₂+4H⁺(in InterMembranous space)
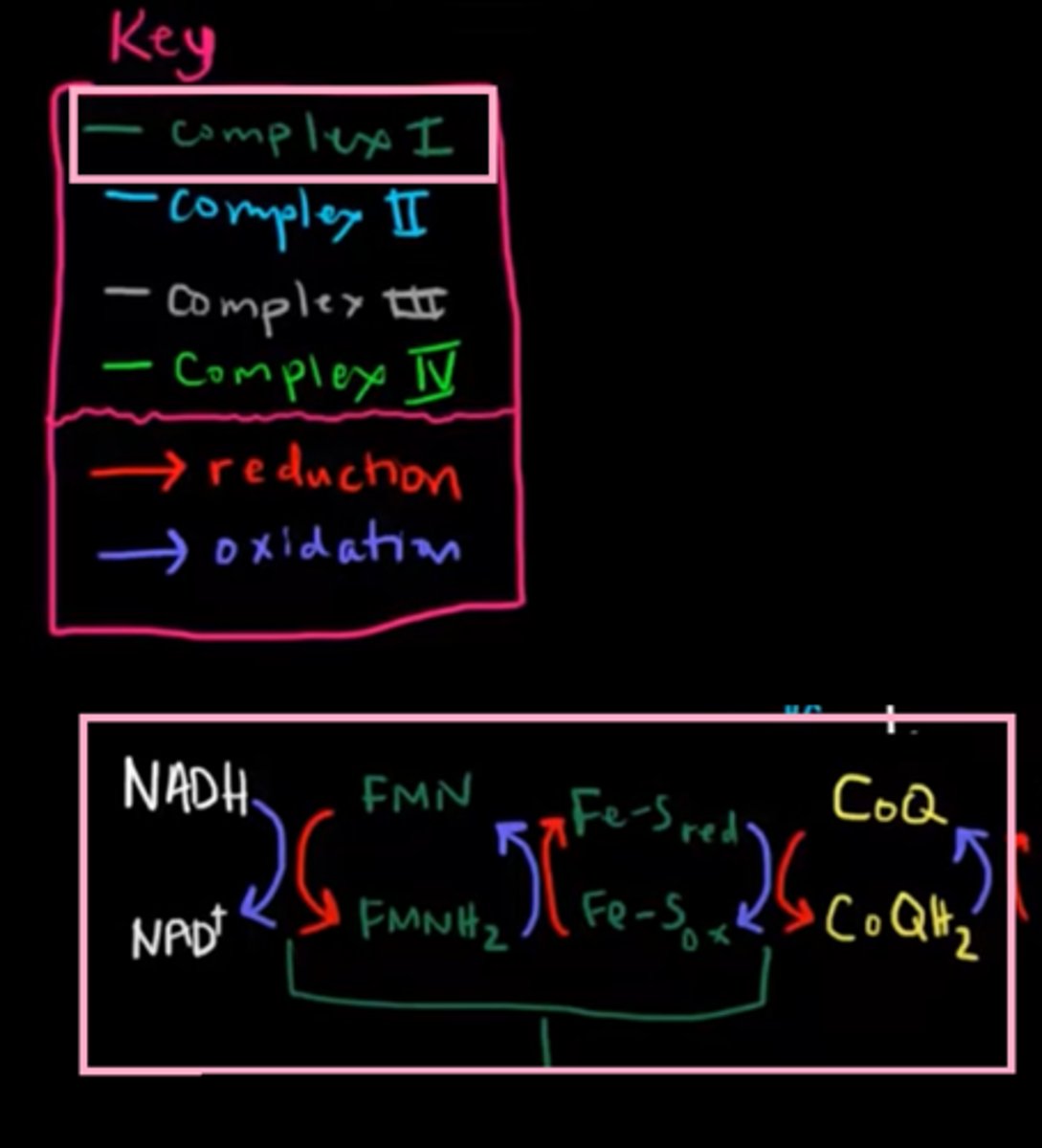
[Complex 2] succinate dehydrogenase
contain prostatic groups:
1. FAD
2. Fe-S clusters (Iron-sulfur clusters)
3. Heme - doesn't transfer e⁻ but it suppress e⁻ leakage from complex II, which can result in the formation of oxygen radicals.
consists of two parts:
1. succinate dehydrogenase - participate in Kreb's cycle, oxidize Succinate to Fumarate & release e⁻ into complex II.
2. this part consists of FAD₂ & UQ, which recieve e⁻ from part 1 of complex II.
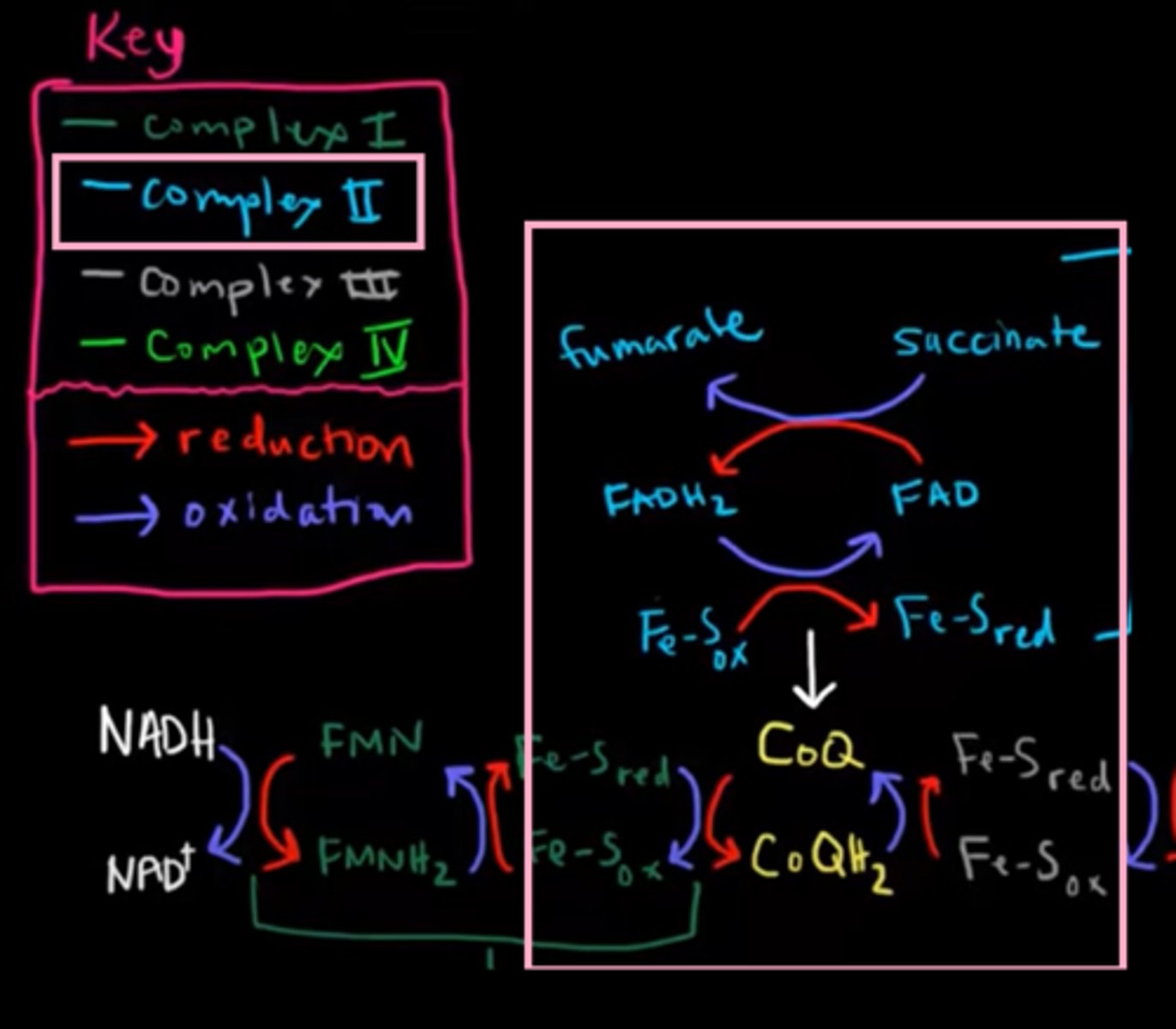
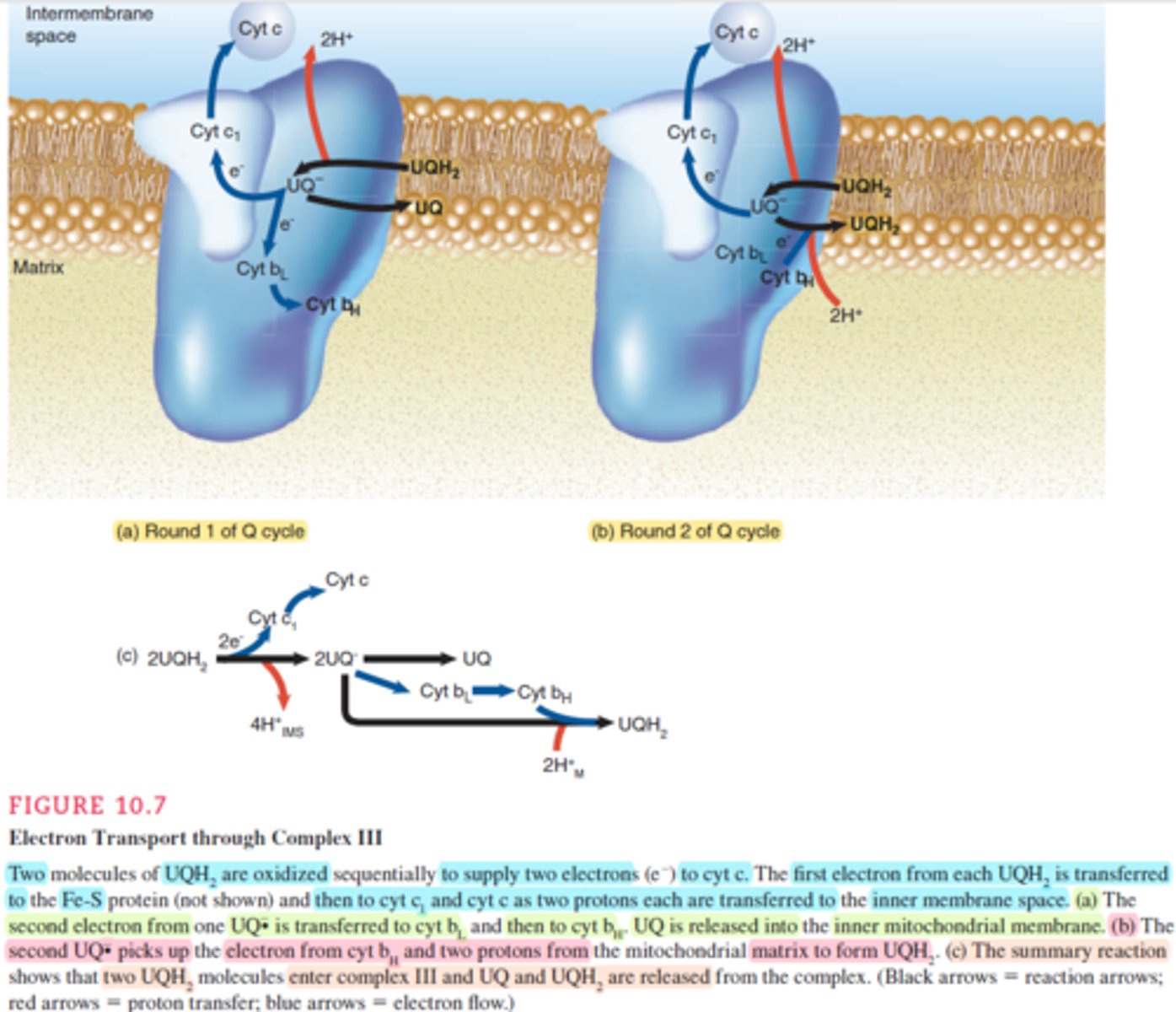
[Complex 3] ubiquinone-cytochrome c oxidoreductase
* This complex is a dimer that transfer e⁻ from reduced UQ to cytochrome C (Cyt C), while using the energy to pump 2H⁺ ions into the intermembranous membrane.
contain prostatic groups & e⁻ carrying molecules:
1. Fe-S clusters (Iron-sulfur clusters)
2. Heme
3. cytochromes (cyt B(l)/B(h)/C₁/C)
passage of e⁻ reffered as two cycles:
* first cycle:
1st UQH₂ enter the complex and transfer one e⁻ to Fe-S clusters -> Cyt C₁ -> Cyt C.
While pumping 2 protons into intermembranous space.
UQH₂ become UQ⁻.
UQ⁻ transfers its second e⁻ to Cyt B₁ -> Cyt B₂.
after releasing the second e⁻, UQ⁻ transformed to UQ and leave into the inner membrane.
* second cycle:
2nd UQH₂ enter the complex and trasnfer one e⁻ to Fe-S clusters -> Cyt C₁ -> Cyt C.
While pumping 2 protons into intermembranous space.
UQH₂ become UQ⁻.
UQ⁻ pickup the e⁻ from Cyt B₂ & 2H⁺ from the matrix to become UQH₂, then it leaves the complex into inetermembranous space.
Note:
UQH₂ = dihydroubiquinone
UQ- = ubisemiquinone
UQ = ubiquinone
[Complex 4] cytochrome oxidase
contain prostatic groups & subunits:
1. Cu(a) & Cu(B) - Cupper ions.
2. Cyt C(a) & Cyt C(a3)
this complex catalyze:
* catalyze the electrons reduction of O₂ to H₂O.
(to form H₂O, 4H⁺ from matrix are used)
* 1 H⁺ (for 1 Cyt C) ion are shuttled through complex 4, so overall 4H⁺ whill be shuttle.
* note - overall complex IV use 8H⁺ from matrix, 4 for forming H₂O and 4 are shuttled to intermembran space.
* e⁻ pass from:
Cyt C -> Cu(a) -> Cyt C(a) -> Cyt C(a3) -> Cu(B) -(Finally to)->O₂
regulation!!!!!
there is ATP-binding regulatory sites on Cyt C & complex IV.
↑[ATP] => decrease in electron transport activity.
![<p>contain prostatic groups & subunits: </p><p>1. Cu(a) & Cu(B) - Cupper ions.</p><p>2. Cyt C(a) & Cyt C(a3)</p><p>this complex catalyze:</p><p>* catalyze the electrons reduction of O₂ to H₂O.</p><p>(to form H₂O, 4H⁺ from matrix are used)</p><p>* 1 H⁺ (for 1 Cyt C) ion are shuttled through complex 4, so overall 4H⁺ whill be shuttle.</p><p>* note - overall complex IV use 8H⁺ from matrix, 4 for forming H₂O and 4 are shuttled to intermembran space. </p><p>* e⁻ pass from:</p><p>Cyt C -> Cu(a) -> Cyt C(a) -> Cyt C(a3) -> Cu(B) -(Finally to)->O₂</p><p>regulation!!!!!</p><p>there is ATP-binding regulatory sites on Cyt C & complex IV.</p><p>↑[ATP] => decrease in electron transport activity.</p>](https://knowt-user-attachments.s3.amazonaws.com/37d9302e-54c8-4e0b-ace1-af535b7594b0.jpg)
Net reaction of the ETC from NADH--> O₂
NADH+H⁺+½O₂+10H⁺(in Matrix) --> NAD⁺+H₂O+10H⁺(in intermembranous space)
what ion passes through F0 component
H+
the component that rotate
gamma spindle
the function of the alpha and beta head
to bind ADP and Pi tightly to synthesis ATP
why when a molecule of FAD2H is processed it generates two ATP molecules while a molecules of NAD2H will generate three ATP
1. NADH transfers electrons to complex I
2. FAD.2H is a reduced coenzyme that transfers electrons to complex II;
3. NADH can only carry one Hydrogen atom whereas FAD.2H can carry two;
4. Therefore FAD.2H can pass more electrons through the electron transport chain which are ultimately used to generate energy in the form of ATP;
similarity and difference between complex I and ubiquinone
Similarity -
protein carrier / transfers electrons;
Difference -
Complex I is a transmembrane protein whereas ubiquinone is a mobile carrier;
where can find the diagram
mitochondria (across the inner mitochondria membrane), oxidative phosphorylation involved