CHAPTER 6 Electronic Structure and Periodic Properties of Elements
1/83
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
84 Terms
electromagnetic radiation
energy transmitted by
waves that have an electric-field component and a
magnetic-field component
electromagnetic spectrum
range of energies that
electromagnetic radiation can comprise, including radio, microwaves, infrared, visible, ultraviolet, X-rays, and gamma rays
wave
oscillation of a property over time or space;
can transport energy from one point to another
constant speed of light (c)
2.998 × 108 m/s
What is wavelength (λ)?
The distance between two consecutive peaks or troughs in a wave.
What determines light's color?
The wavelength and its frequency of the light.
In what units is wavelength measured?
nanometers (nm) or millimeters (mm), m,
frequency (v and nu)
number of wave cycles (peaks or troughs) that pass a specified point in space per unit time
amplitude
extent of the displacement caused by a wave
Hert (Hz)
the unit of frequency, which is the number of cycles per second, s^-1
Brightness
Amplitude is related to the intensity of the wave for light
Loudness
Amplitude related to the intensity of the wave for sound
speed of the wave
= speed of light = wavelength x frequency
Increasing Energy (E)
Increasing frequency and decreasing wavelength
increasing frequency (v)
increasing energy and decreasing wavelength
increasing wavelength
decreasing frequency, energy, mass
interference patterns
A pattern typically consisting of alternating bright and dark fringes; it results from constructive and destructive interference of waves
Standing waves (stationary waves)
remain constrained within some region of space
quantization
A limitation of some property to specific discrete values, not continuous
nodes (n-1)
any point of a standing wave with zero amplitude
blackbody
idealized perfect absorber of all incident electromagnetic radiation; such bodies emit electromagnetic radiation in characteristic continuous spectra called blackbody radiation
Planck's Quantum Theory
assume that the vibrational energies of atoms are not continuous but are quantized
Planck's Equation
E =nhv
E
energy of the vibrating atom, = hc/wavelength
n
integer (1, 2, 3, ...) representing the discrete energy levels
h
Planck's constant (approximately 6.626×10^−34 Js)
photon
smallest possible packet of
electromagnetic radiation, a particle of light
line spectra
the light emission only at specific wavelengths
Atomic Theory
Niels Bohr developed a theory that posits that all matter is composed of tiny particles called atoms.
force of attraction
A force that pulls objects together equals centrifugal force
ground electronic state
state in which the electrons in an atom, ion, or molecule have the lowest energy possible
excited electronic state
state having an energy greater than the ground-state energy
ionization energy
The amount of energy required to remove an electron from an atom (change in E)
ionization energy equation
|change E| = |Ef-Ei| = hv = hc/wavelength
Light
electromagnetic radiation, a form of energy that is made up of oscillating electric and magnetic fields
7 rays of light (low to high energy)
Radio, microwave, infrared, visible light, ultraviolet, x-ray, gamma ray
Red wavelength
620-750 nm
orange wavelength
590-620 nm
yellow wavelength
570-590 nm
green wavelength
500-570 nm
blue wavelength
450-500 nm
violet wavelength
380-450 nm
number of photons equation
#P = Etotal / Ephotons
Avogadro's number
6.022x10^23 ( 1 moles, particles, molecules)
Heisenberg uncertainty principle
It is fundamentally impossible to determine simultaneously and exactly both the momentum and the position of a particle.
wavefunctions
mathematical description of an
atomic orbital that describes the shape of the orbital; it can be used to calculate the probability
of finding the electron at any given location in the orbital, as well as dynamical variables such as the energy and the angular momentum
principle quantum number
number having only specific
allowed values and used to characterize the
arrangement of electrons in an atom
shell
atomic orbitals with the same principal quantum number, n (higher shell, higher energy)
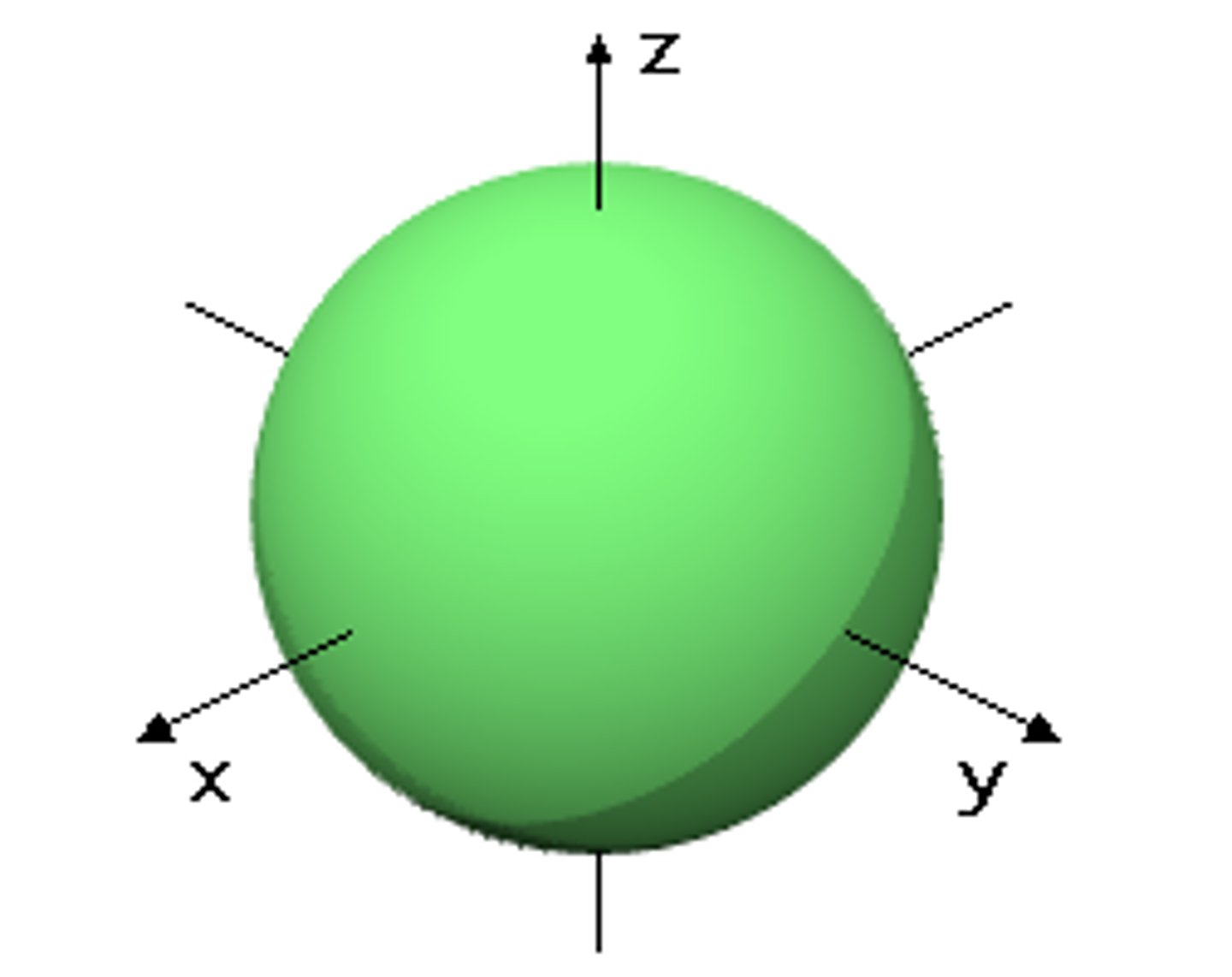
s orbital (sublevel)
a spherical region of space with high electron density, describes orbitals with l= 0
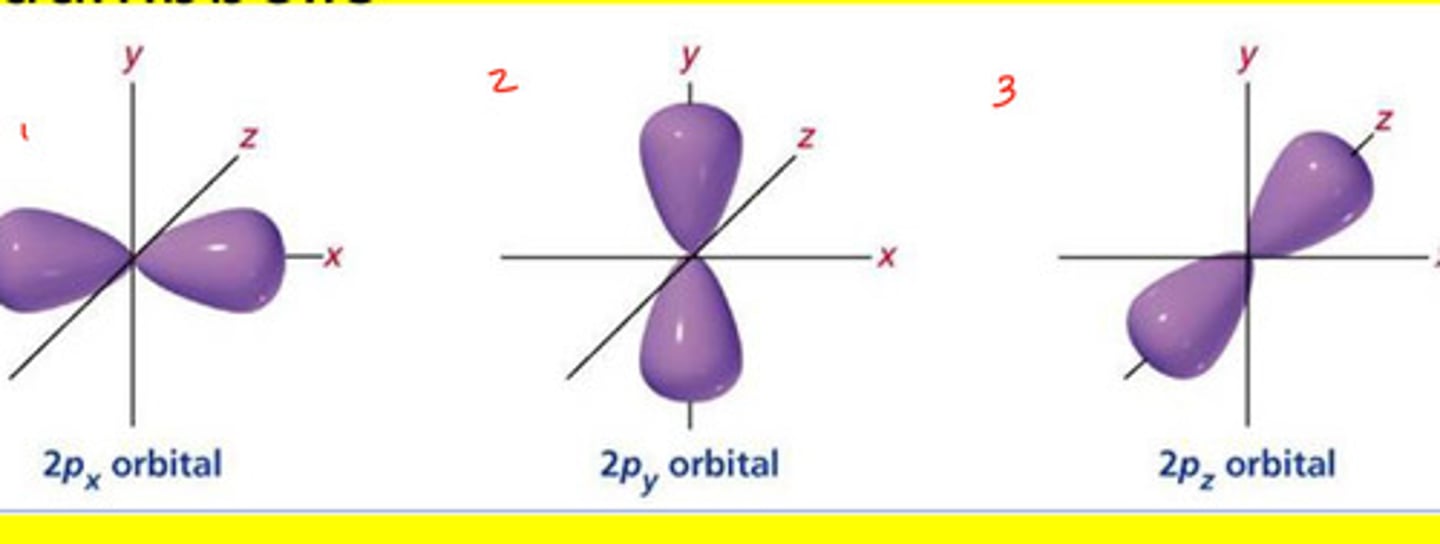
p orbital (p sublevel)
A dumbbell-shaped region of space with high electron density, describes orbitals with l = 1
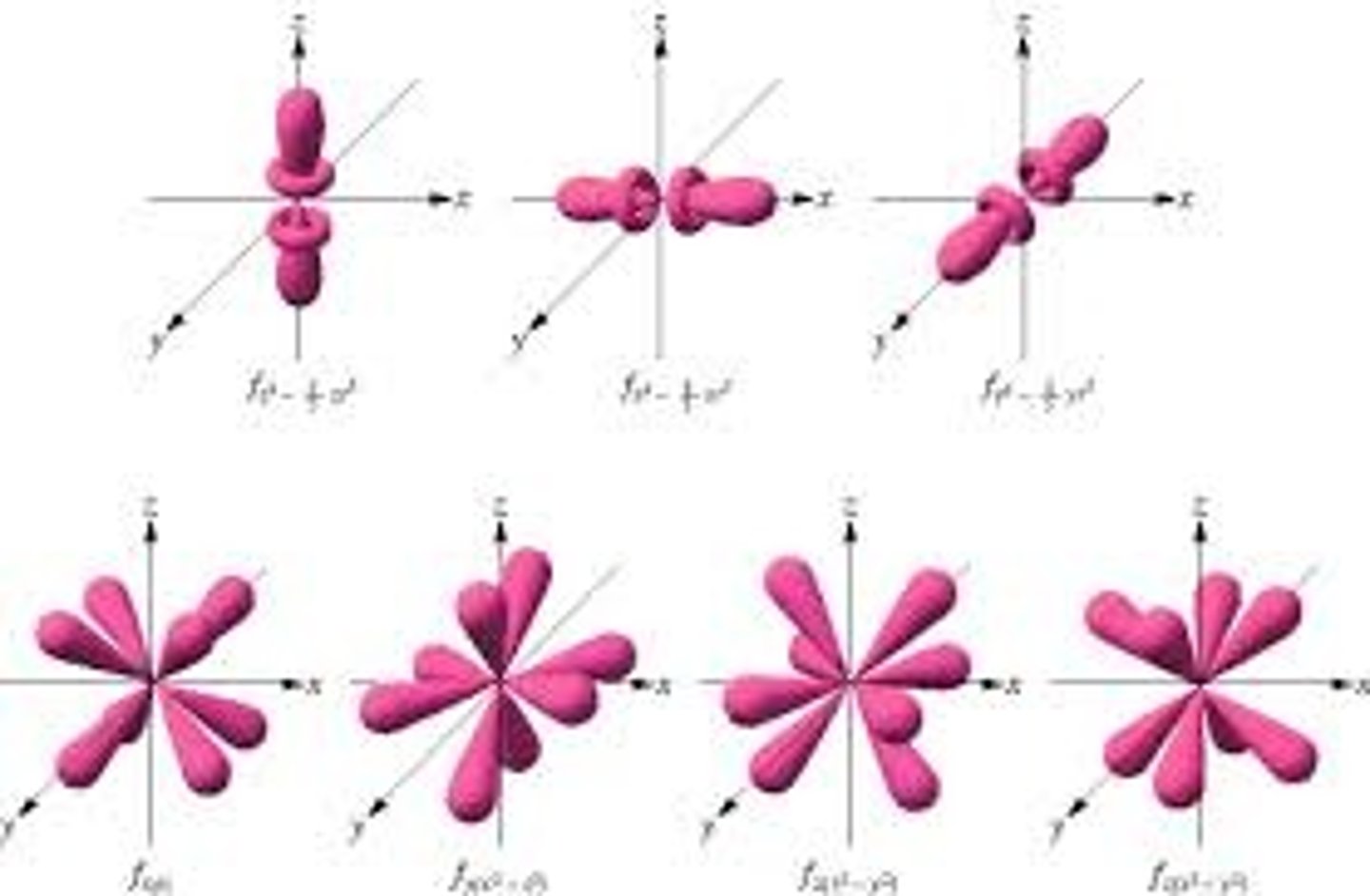
f orbital (f sublevel)
multilobed region of space with high electron density, describes orbitals with l = 3
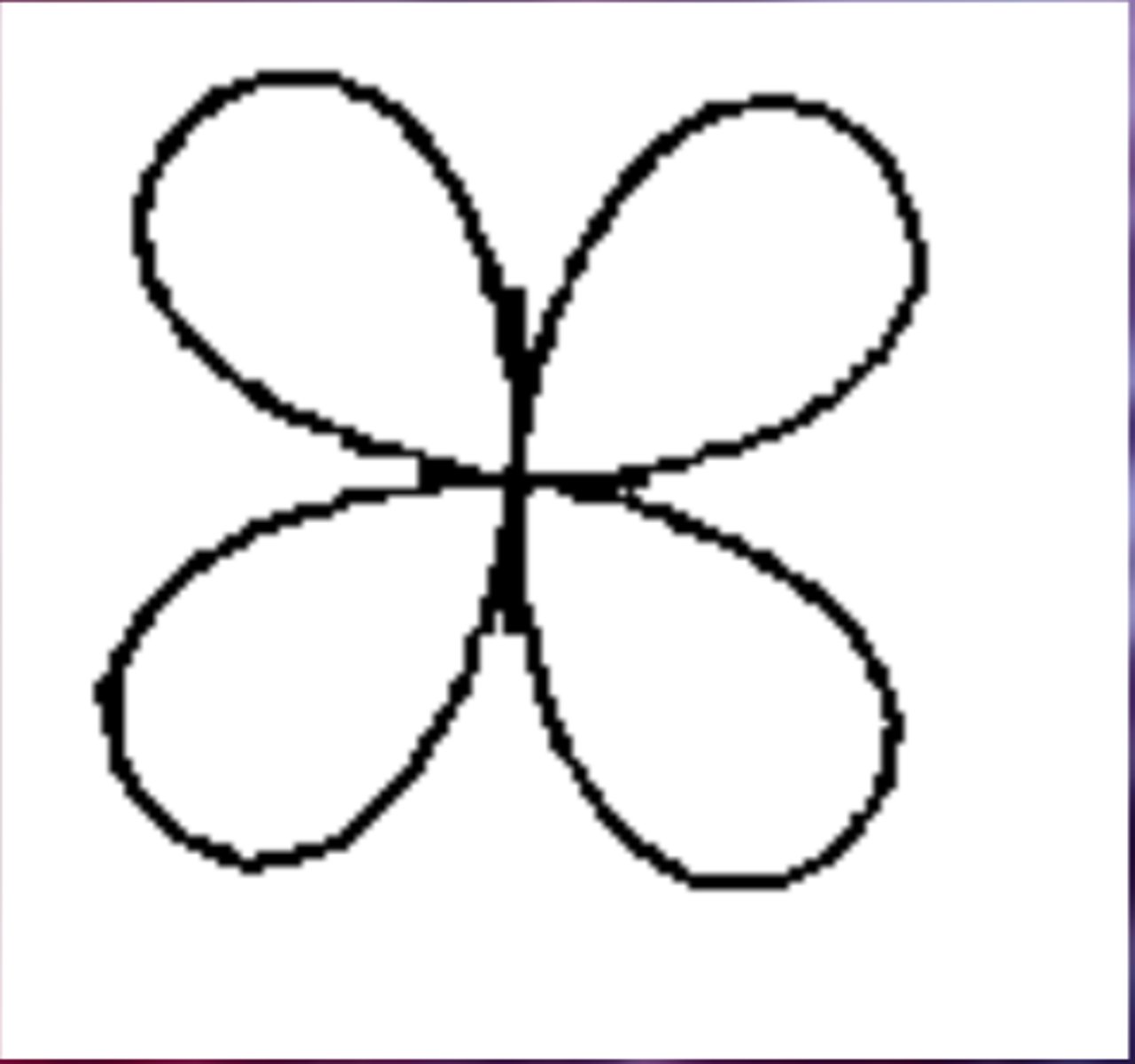
d orbital (d sublevel)
region of space with high electron density that is either four lobed or contains a dumbbell
and torus shape; describes orbitals with l = 2.
principal quantum number
n, shell, the general region for the value of energy for an electron on the orbital
angular momentum or azimuthal quantum number
l, subshell, the shape of the orbital
l equation
0 ≤ l ≤ n - 1
magnetic quantum number
ml, orientation of the orbital
ml equation
- l ≤ ml ≤ l
spin quantum number
ms, direction of the intrinsic quantum "spinning" of the electron, Up (1/2) or down (-1/2)
Pauli Exclusion Principle
No two electrons in the same atom can have exactly the same set of all the four quantum numbers
Electron Configurations
listing that identifies the electron occupancy of an atom's shells and
subshells
What is the order of orbital energy levels from lowest to highest?
s < p < d < f
What does it mean when orbitals are less penetrating?
Less electron density is found near the nucleus.
electron density
A measure of the probability of locating an electron in a particular region of space.
How is electron density calculated
It is equal to the squared absolute value of the wave function ψ.
the Aufbau Principle
A procedure in which the electron configuration of the elements is determined by 'building' them in order of atomic numbers.
How does the Aufbau Principle work?
By adding one proton to the nucleus and one electron to the proper subshell at a time.
valence electrons
The electrons occupying the outermost shell orbital(s) (highest value of n)
core electrons
electrons occupying the inner shell orbitals
covalent/atomic radius
one-half the distance between the nuclei of two identical atoms when they are joined by a covalent bond
atomic radius increase
down and to the left
Ionic Radii
Ion size based on the amount of electrons.
ionic radii increasing
down a column.
What is the order of ionic radii size from largest to smallest?
Anion, neutral, cation.
effective nuclear charge (Zeff)
amount of charge felt by the most recently added electron (Zeff = Z- S (core e-))
isoelectronic
A group of ions or atoms that have
identical electron configurations
first ionization energy (IE1)
energy required to remove the first electron, always endothermic and positive, kj/mol
first ionization energy (IE1) equation
X(g) -> X⁺(g) + e⁻
electron affinity (EA)
energy change associated with
addition of an electron to a gaseous atom or ion, is always negative
electron affinity (EA) equation
X⁺(g) + e⁻ -> X-
electron affinity increasing
up and to the right
ionization energy exceptions
Group 2A (higher, more unstable) to Group 3A and Group 5A (higher) to Group 6A, Ionization energy decreases
Why does removing a full or half-full orbital require more ionization energy?
Because it is more stable and requires more energy to remove an electron.
electron affinity exceptions
1A to 2A (lower) and 4A (lower) to 5A and 8A (lower)
Why does adding to a full or half-full orbital require less electron affinity?
because it is causing more repulsion, making the element bigger, further from the nucleus