10. Pancreatic Hormones
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
Cell Types of the Pancreas
The Islets of Langerhans are ________ cells in the ___________ responsible for ____________ pancreatic hormones
Alpha cells synthesize and secrete __________ and are the most ________
Beta cells synthesize and secrete __________
Delta cells synthesize and secrete ______________
Epsilon cells synthesize and secrete __________
PP/Gamma/F cells cells synthesize and secrete __________ _____________
_____________ ___________ helps transmit __________ for release of pancreatic hormones
Cell Types of the Pancreas
The Islets of Langerhans are endocrine cells in the pancreas responsible for secreting pancreatic hormones
Alpha cells synthesize and secrete glucagon and are the most abundant
Beta cells synthesize and secrete insulin
Delta cells synthesize and secrete somatostatin
Epsilon cells synthesize and secrete ghrelin
PP/Gamma/F cells cells synthesize and secrete pancreatic polypeptides
Autonomic Innervation helps transmit signalsfor release of pancreatic hormones
Insulin Overview
Why is insulin important?
What are its metabolic effects
What is the structure of insulin
Insulin is the most important hormone coordinating the use of fuels
Insulin has anabolic (building) effects such as glycogenesis, fatty acid and protein synthesis
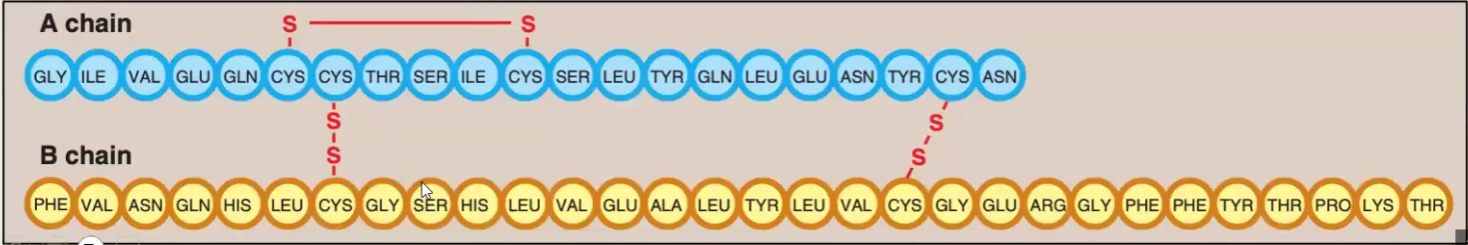
The structure is composed of of 51 AA divided in A and B chains; The chains are held together by 2 disulfide bridges and another intramolecular disulfide bridge is found within the A chain
Insulin Synthesis
What is is the process and what are the differences between the precursors and insulin?
Where is insulin stored and how is it released when needed
How is insulin degraded
Why do we test for C-peptide and not for Insulin levels when we want to know how much insulin is being produced?
In the RER: preproinsulin gets its N-terminal sequence cleaved and becomes proinsulin; In the Golgi A: proinsulin gets the C-peptide cleaved and becomes insulin
Insulin is stored in the cytoplasm and is released by exocytosis
Insulin is degraded by insulinase
We test C-peptide because it has a longer-half life than insulin and is thus a better indicator
Insulin Regulation
Stimulators
Inhibitors
Stimulators: Glucose (proportional relationship with insulin), Amino Acids (Fats too but not nearly as much), gastrointestinal hormones (hunger signals)
Inhibitors: Scarcity of dietary fuels, Illness, Stress/Epinephrine
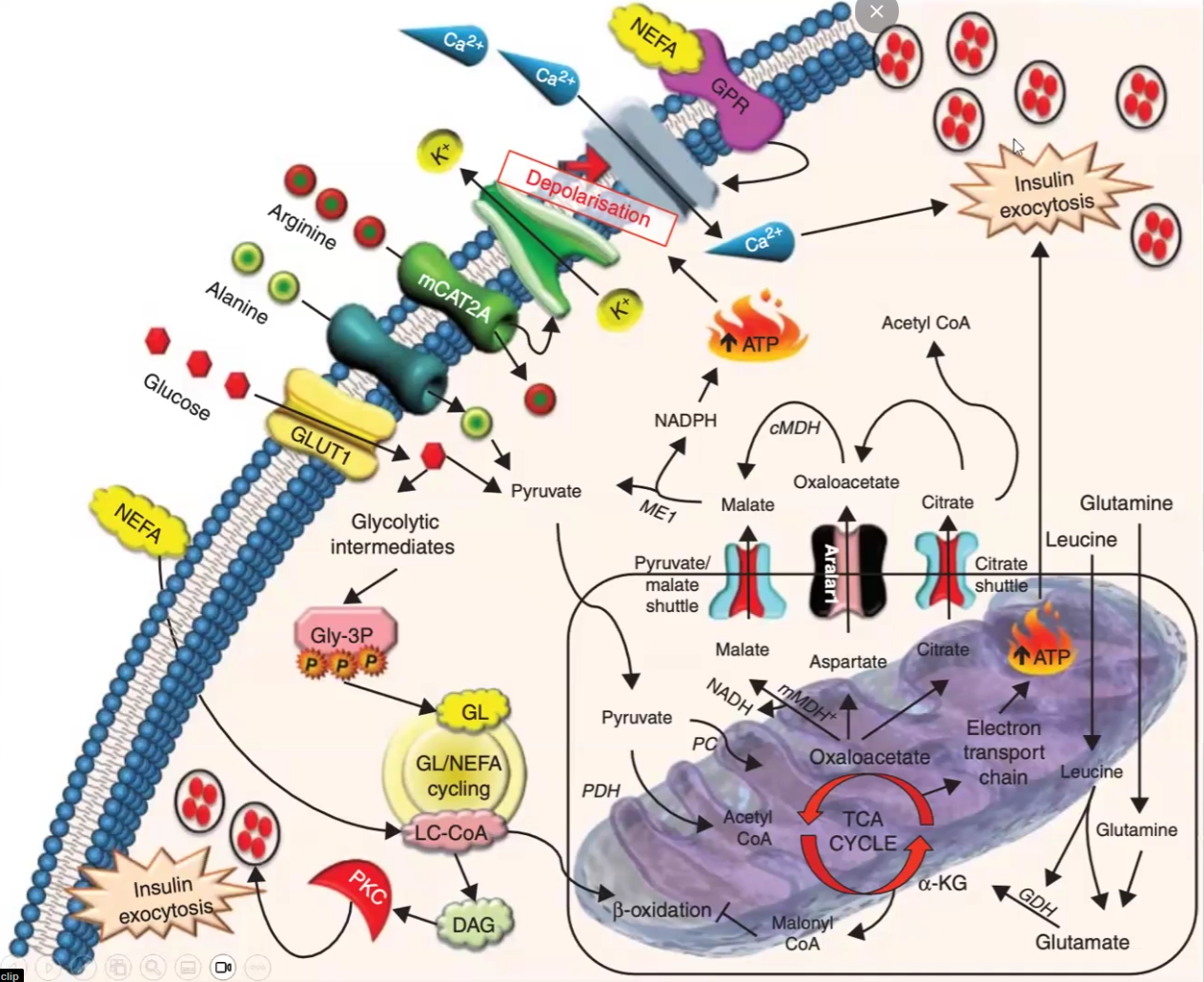
Insulin/Glucagon Secretion from the following starting molecules: mention low glucose and high glucose scenarios
Glucose and Alanine
Arginine
TAG
Insulin during High Glucose
Glucose and Alanine → Pyruvate → ATP → Depolarization of Ca2+ channels → exocytosis of insulin
Arginine → Polarization of K+ channels → Depolarization of Ca2+ channels → exocytosis of insulin
TAG → many ways to produce energy → ATP → Depolarization of Ca2+ channels → exocytosis
Glucagon during high glucose
Blocks channels and does not exocytose glucagon
Insulin during Low Glucose
Blocks channels and does not exocytose
Glucagon during Low Glucose (same effect as insulin during high glucose but i am not gonna write it again)
Channels open up due to depolarization and it leads to glucagon exocytosis
Insulin Mechanism of Action
Insulin-dependent Transport of Glucose Mechanism
What are the insulin-independent tissues
Insulin Mechanism of Action
Insulin binds tyrosine kinase receptor at the alpha subunit
The binding causes autophosphorylation of the beta subunit of the tyrosine kinase
Phosphorylated tyrosine kinase phosphorylates Insulin Receptor Substrates (IRS) → causes systemic effects
Insulin-dependent Transport of Glucose Mechanism
Insulin binding promotes recruitment of glucose transporters from storage pool
Glucose transporters fuse with the membrane and let glucose pass through
When insulin is absent they move out the cell membrane and return to the storage pool
They are very niche organs such as the brain, then leukocytes, erythrocytes, lens and cornea of eye and liver
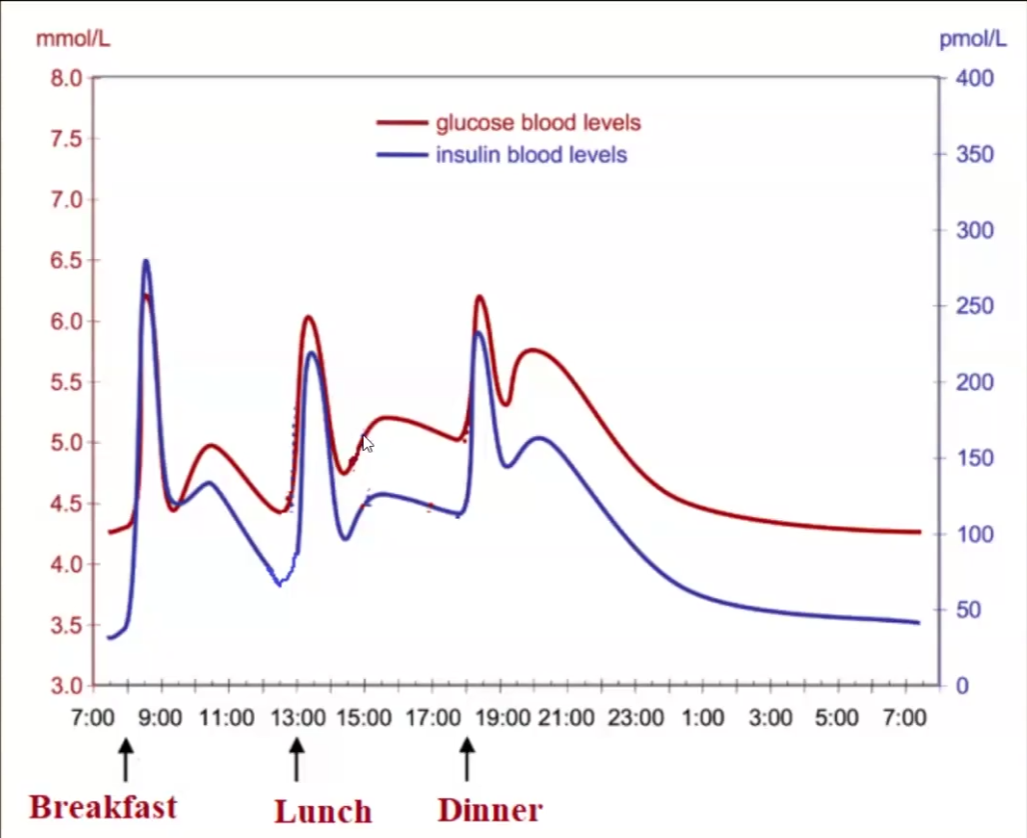
Insulin vs Blood Glucose levels Graph
Insulin is only higher than glucose during the morning/breakfast → most important meal for regulating insulin
The res of the day insulin and glucose should be almost equal
Glucagon Overview
Why is glucagon important?
What are its metabolic effects?
What is the structure of glucagon?
How is the synthesis of glucagon in comparison to insulin
Because it opposes the effect of insulin during the fasting state by maintaining blood glucose levels
Activates glycogenolysis, gluconeogenesis (only in liver, muscle does not have glucagon receptors), fatty acid oxidation and ketogenesis
Glucagon is a a single polypeptide chain of 29 amino acids
It is pretty much the same thing except the name changes to glucagon → preproglucagon and proglucagon
Glucagon Regulation
Stimulants
Inhibitors
Stimulants:
Low blood glucose due to prolonged fasting (>2hrs)
Amino acids: also stimulate insulin but in this case availability of AA will allow gluconeogenesis to take place
Epinephrine, cortisol
Inhibitors:
Elevated blood glucose
Ingestion of carb-rich meal
Insulin
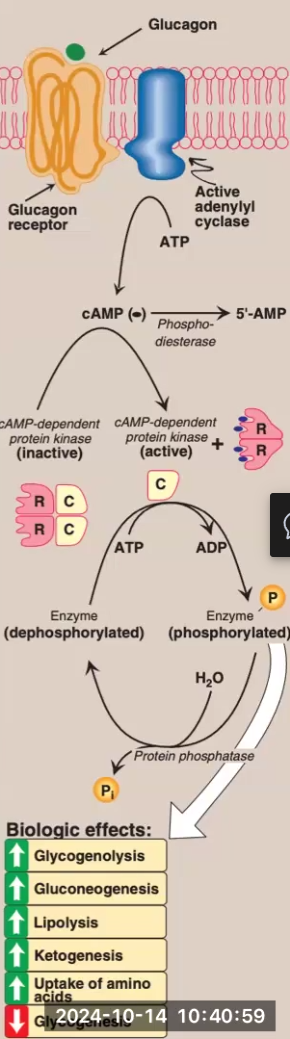
Glucagon Mechanism of Action
Glucagon binds to G protein-coupled receptors on hepatocytes
Remember that muscle only has epinephrine receptors, not glucagon receptors
Glucagon binding activates adenyl cyclase
Adenyl cyclase causes a rise in AMP → cAMP
Rise in cAMP activates cAMP-dependent protein kinase
cAMP-dependent protein kinase phosphorylates enzymes that give various metabolic effects (glycogenolysis, gluconeogenesis, etc.)
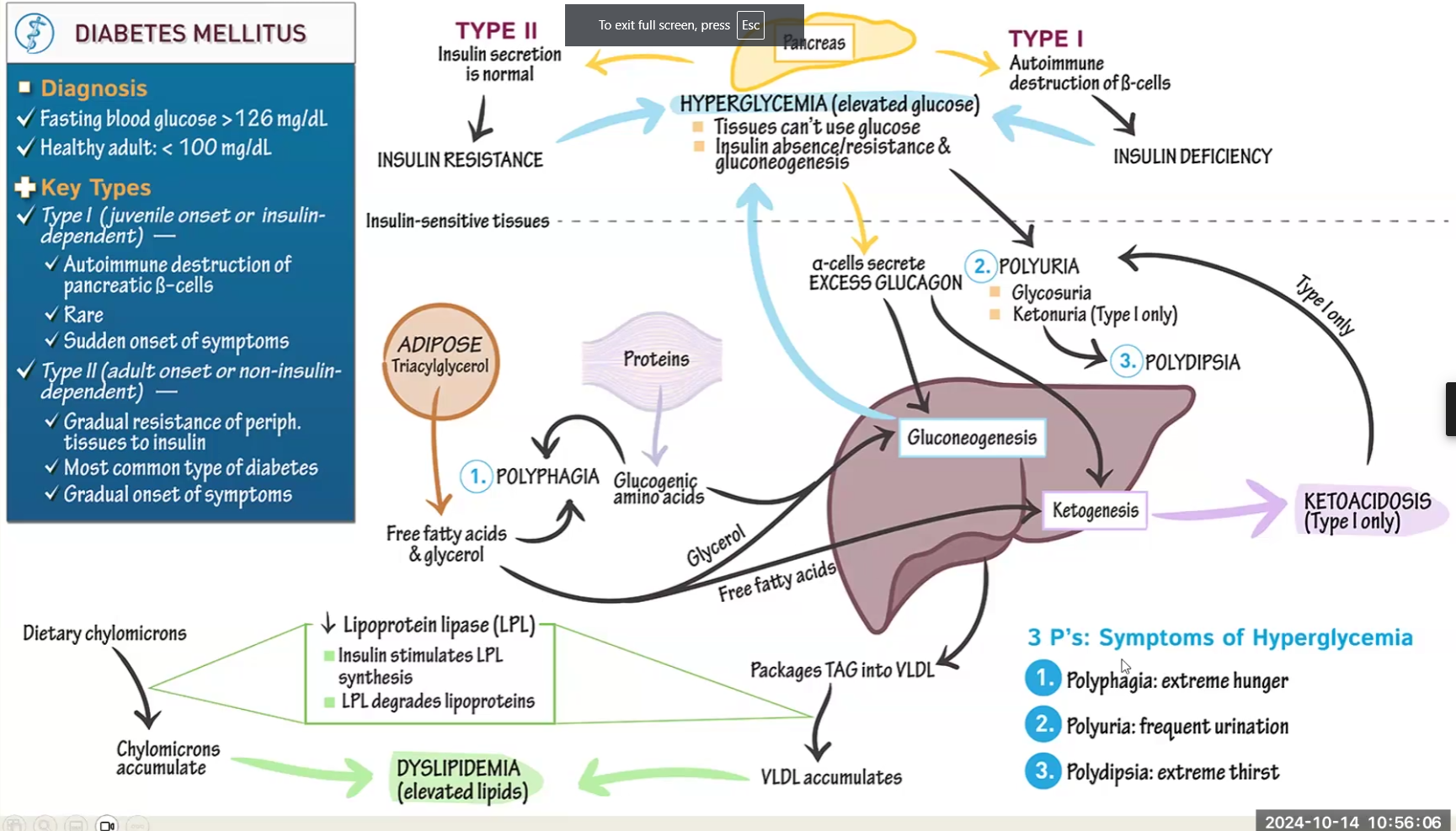
Diabetes Mellitus
Explain the Disease
What is the problem
What process acts to make up for it
What is degraded to make up for it
What enzyme activity is decreased and what are the consequences
Symptoms
Difference between Type I and Type II
Diabetes causes tissues to not be able to use glucose
Gluconeogenesis and ketogenesis are higher than normal to try and make up
Metformin drug used to regulate -
Degradation of adipose into TAG and degradation of protein also present to try to make up
Lipoprotein Lipase is decreased because it requires insulin activation, therefore chylomicrons accumulate → Results in Dyslipidemia → elevated TAG, elevated cholesterol, etc.
Symptoms:
Polyphagia: extreme hunger
Polydipsia: extreme thirst
Polyuria: frequent urination
Type I:
autoimmune disease present from childhood
Destruction of beta cells, no insulin
Increased Ketogenesis may lead to ketoacidosis
Type 2:
acquired, insulin secretion is normal but cells are resistant
Metformin drug used to regulate gluconeogenesis
Other hormonal involvement (don’t have to know specifics, just that there are other important hormones)
Ghrelin (from epsilon cells)→ Hunger signals
Somatostatin (from delta cells)
Gastrin, Secretin, CCK, GIP, GLP-1,GH, Cortisol