Biological molecules
0.0(0)
Card Sorting
1/121
Earn XP
Description and Tags
Last updated 9:14 AM on 5/29/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
122 Terms
1
New cards
Water composition
\-2 hydrogen atoms
\-1 oxygen atom
\-Joined by covalent bonds
\-Triangular shape with unusual distribution of charges
\-1 oxygen atom
\-Joined by covalent bonds
\-Triangular shape with unusual distribution of charges
2
New cards
Charge of water
\-Overall neutral but uneven
\-Oxygen has negative charge
\-Hydrogen has positive charge
\-Oxygen has negative charge
\-Hydrogen has positive charge
3
New cards
Oxygen draws
Negative electrons
4
New cards
Polar molecule
Molecule with negative and positive charge eg. water
5
New cards
Dipole
Separation of charge due to electrons in covalent bonds being unevenly shared
6
New cards
Hydrogen bonding
\-Form between water molecules
\-Due to polarity of water, they form between the positively and negatively charged regions of adjacent molecules
\-Negative oxygen attracts positive hydrogen, so they flow together
\-Bonds are strong when in large numbers
\-Contribute to water molecules properties
\-Due to polarity of water, they form between the positively and negatively charged regions of adjacent molecules
\-Negative oxygen attracts positive hydrogen, so they flow together
\-Bonds are strong when in large numbers
\-Contribute to water molecules properties
7
New cards
Water properties
\-Solvent
\-High specific heat capacity
\-High latent heat of vaporisation
\-Less dense as a solid
\-High cohesion and adhesion
\-High specific heat capacity
\-High latent heat of vaporisation
\-Less dense as a solid
\-High cohesion and adhesion
8
New cards
Water properties: solvent
\-Many ions and polar molecules will dissolve in water because its polar (eg. glucose and sodium chloride)
\-Allows chemical reactions to occur within cells, dissolved solutes are free to move and more chemically reactive
\-Metabolites can be transported efficiently
\-Ideal transport medium
\-Allows chemical reactions to occur within cells, dissolved solutes are free to move and more chemically reactive
\-Metabolites can be transported efficiently
\-Ideal transport medium
9
New cards
Water properties: high specific heat capacity
\-4200 J/kg°C
\-Large amount of energy needed to raise waters temperature
\-Due to many hydrogen bonds present in water it takes a lot of thermal energy to break them and build them, so temp does not fluctuate greatly
\-This provides suitable habitats for marine life and means optimal temps are maintained within cells and bodies (eg. enzyme activity and water in blood plasma)
\-Large amount of energy needed to raise waters temperature
\-Due to many hydrogen bonds present in water it takes a lot of thermal energy to break them and build them, so temp does not fluctuate greatly
\-This provides suitable habitats for marine life and means optimal temps are maintained within cells and bodies (eg. enzyme activity and water in blood plasma)
10
New cards
Water properties: high latent heat of vaporisation
\-Large amount of thermal energy is needed to be absorbed to break hydrogen bonds and evaporate (change state from liquid to gas)
\-Means only a little water is needed to evaporate for organism to lose great amount of heat, its a coolant
\-Eg. evaporation of water as sweat
\-Means only a little water is needed to evaporate for organism to lose great amount of heat, its a coolant
\-Eg. evaporation of water as sweat
11
New cards
Water properties: less dense as a solid
\-Ice is less dense than liquid water due to hard to break hydrogen bonds, so it floats
\-Creates insulating layers in bodies of water, keeping water underneath a constant temp, helping organisms survive
\-Creates insulating layers in bodies of water, keeping water underneath a constant temp, helping organisms survive
12
New cards
Water properties: high cohesion and adhesion
\-Water molecules stick together due to polarity, so water can flow to transport substances
\-Water molecules bond to each other, allowing water to move up xylem in transpiration
\-Important in plants water movement
\-Water molecules bond to each other, allowing water to move up xylem in transpiration
\-Important in plants water movement
13
New cards
The essential roles of water due to what properties
Water molecules polarity and hydrogen bonds
14
New cards
Key molecules needed for organisms to function
\-Carbs
\-Proteins
\-Lipids
\-Nucleic acids
\-Water
\-Proteins
\-Lipids
\-Nucleic acids
\-Water
15
New cards
Monomer
Single small molecule
Can bond together to form polymers
Can bond together to form polymers
16
New cards
Dimer
2 monomers joined by a condensation reaction
17
New cards
Polymer
Molecules made from many monomers joined by covalent bonds in a condensation reaction
18
New cards
Polymerisation
Process of joining monomers together to make a polymer
19
New cards
Covalent bonding
\-Sharing of 2+ electrons between 2 atoms
\-Very stable as high energy is needed to break the bonds
\-Electrons shared equally = non polar
\-Electrons shared unequally = polar
\-Very stable as high energy is needed to break the bonds
\-Electrons shared equally = non polar
\-Electrons shared unequally = polar
20
New cards
Condensation reaction
2 molecules joined with a covalent bond to form a polymer
\-Release 1 molecule of water
\-Release 1 molecule of water
21
New cards
Hydrolysis reaction
The breaking of a covalent bond between 2 molecules using 1 molecule of water (water is added)
22
New cards
Macromolecules
Very large molecules with high molecular mass
23
New cards
Covalent bond in carbohydrates
Glycosidic bond
24
New cards
Monomer of carbohydrates
Monosaccharides (glucose and ribose)
25
New cards
Covalent bond in proteins
Peptide bond
26
New cards
Monomer of proteins
Amino acids
27
New cards
Covalent bond in lipids
Ester bond
28
New cards
Monomer of lipids
Fatty acids and glycerol
29
New cards
Covalent bond in nucleic acids
Phosphodiester bond
30
New cards
Monomer of nucleic acids
Nucleotides
31
New cards
What makes biological molecules organic compounds
Them containing the chemical elements carbon and hydrogen
32
New cards
Why are carbon atoms key to organic compounds
\-Each can form 4 covalent bonds (making compounds very stable)
\-Can form the bonds with oxygen, nitrogen and sulfur
\-Can form straight chains, branched chains and rings
\-Can form the bonds with oxygen, nitrogen and sulfur
\-Can form straight chains, branched chains and rings
33
New cards
Chemical elements of carbohydrates and lipids
C, H, O
(Proportion of O in lipids is lower)
(Proportion of O in lipids is lower)
34
New cards
Chemical elements of proteins
C, H, O, N, S
35
New cards
Chemical elements of nucleic acids
C, H, O, N, P
36
New cards
Carbohydrates
\-1C:2H:1O
\-C*x* (H2O)*y*
\-3 types are monosaccharides, disaccharides and polysaccharides
\-Source and store of energy
\-Structurally important
\-C*x* (H2O)*y*
\-3 types are monosaccharides, disaccharides and polysaccharides
\-Source and store of energy
\-Structurally important
37
New cards
Monosaccharides
\-Simplest carbohydrate
\-Made of 1 simple sugar monomer
\-Reducing sugars
\-Includes glucose and ribose (and galactose and fructose)
\-Made of 1 simple sugar monomer
\-Reducing sugars
\-Includes glucose and ribose (and galactose and fructose)
38
New cards
Isomer
\-Organic molecules that have the same molecular formula but different structures
\-Results in different properties
\-Eg. glucose
\-Results in different properties
\-Eg. glucose
39
New cards
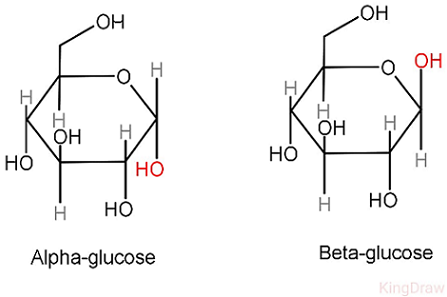
Glucose
\-Has 6 carbon atoms (hexose)
\-C6 H12 O6
\-Most common monosaccharide
\-Main energy source in animals and plants
\-Main substrate used in respiration, releases energy for ATP production
\-Its soluble and so easy to transport in water
\-Exists as alpha and beta glucose (an isomer)
\-C6 H12 O6
\-Most common monosaccharide
\-Main energy source in animals and plants
\-Main substrate used in respiration, releases energy for ATP production
\-Its soluble and so easy to transport in water
\-Exists as alpha and beta glucose (an isomer)
40
New cards
Alpha glucose (α)
\-OH group below the ring
\-In starch and glycogen (polysaccharides)
\-In maltose and sucrose (disaccharides)
\-In starch and glycogen (polysaccharides)
\-In maltose and sucrose (disaccharides)
41
New cards
Beta glucose (β)
\-OH group above the ring
\-In cellulose (polysaccharide)
\-In cellulose (polysaccharide)
42
New cards
Hexose
Monosaccharide with 6 carbon atoms
eg. glucose
eg. glucose
43
New cards
Pentose
Monosaccharide with 5 carbon atoms
eg. ribose and deoxyribose
eg. ribose and deoxyribose
44
New cards
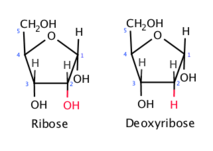
Ribose
\-Has 5 carbon atoms (pentose)
\-Ribose and deoxyribose found in nucleotides that make up RNA and DNA
\-The oxygen atom at C2 is lost in deoxyribose
\-A monosaccharide
\-Ribose and deoxyribose found in nucleotides that make up RNA and DNA
\-The oxygen atom at C2 is lost in deoxyribose
\-A monosaccharide
45
New cards
OIL RIG
\-Oxidation Is Loss (of electrons)
\-Reduction Is Gain (of electrons)
\-Reduction Is Gain (of electrons)
46
New cards
Disaccharides
\-Complex sugars
\-2 monosaccharides joined by a glycosidic bond in a condensation reaction
\-Includes maltose, lactose and sucrose
\-2 monosaccharides joined by a glycosidic bond in a condensation reaction
\-Includes maltose, lactose and sucrose
47
New cards
Maltose
\-Alpha glucose and alpha glucose
\-Sugar formed in production and breakdown of starch
\-A disaccharide
\-1,4 glycosidic bond
\-Sugar formed in production and breakdown of starch
\-A disaccharide
\-1,4 glycosidic bond
48
New cards
Sucrose
\-Alpha glucose and fructose
\-Main sugar produced in plants
\-A disaccharide
\-1,2 glycosidic bond
\-Main sugar produced in plants
\-A disaccharide
\-1,2 glycosidic bond
49
New cards
Lactose
\-Alpha glucose and galactose
\-Sugar found only in milk
\-A disaccharide
\-Sugar found only in milk
\-A disaccharide
50
New cards
Glycosidic bond
\-An oxygen bridge
\-Formed by condensation reactions (water molecule removed)
\-Since each bond is catalysed by enzymes specific to which OH groups are interacting, there are different types of glycosidic bonds (1,4 / 1,2)
\-Formed by condensation reactions (water molecule removed)
\-Since each bond is catalysed by enzymes specific to which OH groups are interacting, there are different types of glycosidic bonds (1,4 / 1,2)
51
New cards
Breakage of the glycosidic bond
Bond is broken when water is added (hydrolysis reaction)
52
New cards
Synthesis of disaccharides and polysaccharides
Hydrogen atom bonds to a hydroxyl group (OH) on another monosaccharide, releasing a water molecule (condensation)
Strong covalent bond formed (glycosidic bond)
Strong covalent bond formed (glycosidic bond)
53
New cards
Breakdown of disaccharides and polysaccharides
A water molecule reacts with the glycosidic bond, breaking it apart (hydrolysis)
54
New cards
Polysaccharides
2+ monosaccharides joined by glycosidic bonds through condensation reactions
\-Starch, glycogen and cellulose molecules
\-Macromolecules
\-Chains formed (branched/unbranched, folded/straight)
\-Starch, glycogen and cellulose molecules
\-Macromolecules
\-Chains formed (branched/unbranched, folded/straight)
55
New cards
Starch
\-A polysaccharide
\-Main energy storage for plants
\-Excess glucose stored (when plants need energy, the starch is broken down to release glucose)
\-Formed from 2 polysaccharides of alpha glucose (amylose and amylopectin)
\-Coiling and folding makes its structure compact and stable
\-Insoluble in water (water can’t enter the cell) = more storage and no effect to osmotic properties of cell
\-Main energy storage for plants
\-Excess glucose stored (when plants need energy, the starch is broken down to release glucose)
\-Formed from 2 polysaccharides of alpha glucose (amylose and amylopectin)
\-Coiling and folding makes its structure compact and stable
\-Insoluble in water (water can’t enter the cell) = more storage and no effect to osmotic properties of cell
56
New cards
Amylose
\-20% of starch
\-Long, unbranched chain of alpha glucose
\-Linked with 1,4 glycosidic bonds that give a coiled structure
\-Compact and good for storage
\-Long, unbranched chain of alpha glucose
\-Linked with 1,4 glycosidic bonds that give a coiled structure
\-Compact and good for storage
57
New cards
Amylopectin
\-80% of starch
\-Long, branched chain of alpha glucose
\-Linked with 1,4 glycosidic bonds
\-Side branches linked with 1,6 glycosidic bonds (allow enzymes to get at the bonds easier, releasing glucose quickly)
\-Long, branched chain of alpha glucose
\-Linked with 1,4 glycosidic bonds
\-Side branches linked with 1,6 glycosidic bonds (allow enzymes to get at the bonds easier, releasing glucose quickly)
58
New cards
Glycogen
\-A polysaccharide
\-Main energy storage for animals
\-Excess glucose stored as glycogen granules
\-Short chains of alpha glucose linked with 1,4 glycosidic bonds
\-Highly branched with 1,6 glycosidic bonds (similar to amylopectin)
\-Lots of branches mean stored glucose can be released quickly
\-Smaller chains mean it hydrolyses quicker
\-Very compact molecule = good for storage
\-Main energy storage for animals
\-Excess glucose stored as glycogen granules
\-Short chains of alpha glucose linked with 1,4 glycosidic bonds
\-Highly branched with 1,6 glycosidic bonds (similar to amylopectin)
\-Lots of branches mean stored glucose can be released quickly
\-Smaller chains mean it hydrolyses quicker
\-Very compact molecule = good for storage
59
New cards
Cellulose
\-A polysaccharide
\-Major component of cell wall in plants
\-Long, unbranched chains of beta glucose linked with 1,4 glycosidic bonds
\-Each chain linked to each other by hydrogen bonds, forming microfibrils (strong fibers that are tightly crossed)
\-Every alternate beta glucose molecule is inverted, so even more hydrogen bonds can form, making it very strong for structural support
\-Major component of cell wall in plants
\-Long, unbranched chains of beta glucose linked with 1,4 glycosidic bonds
\-Each chain linked to each other by hydrogen bonds, forming microfibrils (strong fibers that are tightly crossed)
\-Every alternate beta glucose molecule is inverted, so even more hydrogen bonds can form, making it very strong for structural support
60
New cards
Carbohydrate summary
Monosaccharides: single molecule, soluble, function of energy source eg. glucose and ribose
Disaccharides: 2 molecules, soluble, function of energy release and storage eg. maltose, lactose, sucrose
Polysaccharides: 2+ molecules, insoluble, function of energy storage and structural support eg. starch, glycogen, cellulose
Disaccharides: 2 molecules, soluble, function of energy release and storage eg. maltose, lactose, sucrose
Polysaccharides: 2+ molecules, insoluble, function of energy storage and structural support eg. starch, glycogen, cellulose
61
New cards
Macromolecules
Proteins, carbs, lipids, nucleic acids
62
New cards
Lipids
\-Act as an energy source, store and insulating layer
\-All contain C, H and O
\-Triglycerides and phospholipids
\-All contain C, H and O
\-Triglycerides and phospholipids
63
New cards
Triglycerides
\-Fats and oils formed from a condensation reaction between fatty acids and glycerol with an ester bond
\-3 fatty acid molecules combine with 1 glycerol molecule
\-3 fatty acid molecules combine with 1 glycerol molecule
64
New cards
Fatty acid molecules
\-Form triglycerides
\-Have long tails of hydrocarbon chains
\-Carboxyl group (COOH) at one end (this reacts with the hydroxyl (OH) group on the glycerol molecule)
\-Methyl group (CH3) at the other end makes the chain hydrophobic (repels water molecules)
\-Have long tails of hydrocarbon chains
\-Carboxyl group (COOH) at one end (this reacts with the hydroxyl (OH) group on the glycerol molecule)
\-Methyl group (CH3) at the other end makes the chain hydrophobic (repels water molecules)
65
New cards
Glycerol molecules
\-Form triglycerides
\-Have 3 hydroxyl (OH) groups (3 carboxyl groups on the fatty acid molecules can bond to the glycerol by 3 condensation reactions)
\-Have 3 hydroxyl (OH) groups (3 carboxyl groups on the fatty acid molecules can bond to the glycerol by 3 condensation reactions)
66
New cards
Synthesis of a triglyceride
3 condensation reactions occur between the 3 hydroxyl groups (on 1 glycerol molecule) and 3 carboxyl groups (on 3 fatty acids molecules)
3 water molecules are lost and 3 ester bonds are formed (esterification)
Methyl group on the end opposite the carboxyl groups make the fatty acid hydrophobic
3 water molecules are lost and 3 ester bonds are formed (esterification)
Methyl group on the end opposite the carboxyl groups make the fatty acid hydrophobic
67
New cards
Breakdown of triglycerides
Hydrolysis reaction occurs using 3 water molecules to break down the 3 ester bonds
68
New cards
Saturated fatty acids
\-No double bond between their carbon atoms
\-Fats
\-Higher melting point
\-Fats
\-Higher melting point
69
New cards
Unsaturated fatty acids
\-Have at least 1 double bond between their carbon atoms
\-Oils
\-Lower melting point
\-Chain kinks
\-Oils
\-Lower melting point
\-Chain kinks
70
New cards
Features of triglycerides
\-Efficient energy stores (long hydrocarbon tails contain lots of chemical energy which is released when broken down)
\-Insoluble (hydrophobic fatty acid tails prevent cell’s water potential to change)
\-Poor conductor of heat, so good for insulation (eg. aquatic animals like whales)
\-Provides protection around vital organs like kidneys
\-Insoluble (hydrophobic fatty acid tails prevent cell’s water potential to change)
\-Poor conductor of heat, so good for insulation (eg. aquatic animals like whales)
\-Provides protection around vital organs like kidneys
71
New cards
Phospholipids
\-Similar to triglycerides, but 1 fatty acid is replaced by a phosphate group
\-1 glycerol molecule, 2 fatty acid molecules (hydrophobic), 1 phosphate group (hydrophilic)
\-Found in all cell membranes (make up the phospholipid bilayer)
\-1 glycerol molecule, 2 fatty acid molecules (hydrophobic), 1 phosphate group (hydrophilic)
\-Found in all cell membranes (make up the phospholipid bilayer)
72
New cards
Cholesterol
\-Hydrocarbon ring structure attached to a hydrocarbon tail in a phospholipid
\-Small size and flattened shape, so can fit in between phospholipids in the cell membrane
\-They regulate the fluidity and strength of a cell membrane
\-At high temps, they bind to tails to pack more closely = rigid membrane
\-At low temps, they prevent tails from packing too tight = increased fluidity
\-Small size and flattened shape, so can fit in between phospholipids in the cell membrane
\-They regulate the fluidity and strength of a cell membrane
\-At high temps, they bind to tails to pack more closely = rigid membrane
\-At low temps, they prevent tails from packing too tight = increased fluidity
73
New cards
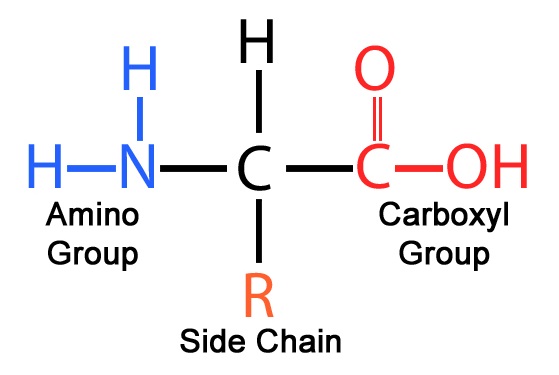
Structure of amino acids
\-Central carbon atom (C)
\-Carboxyl group (COOH) to the right of C
\-Amine group (NH2) to the left of C
\-R group attached to C
\-Hydrogen atom attached to C
\-Carboxyl group (COOH) to the right of C
\-Amine group (NH2) to the left of C
\-R group attached to C
\-Hydrogen atom attached to C
74
New cards
Proteins
\-Basic materials for cell growth, repair and replacement
\-Polymer, made up of amino acid monomers
\-Made up of polypeptides
\-All contain C, H, O, N, S
\-Polymer, made up of amino acid monomers
\-Made up of polypeptides
\-All contain C, H, O, N, S
75
New cards
Dipeptide
2 amino acids join together
76
New cards
Polypeptide
2+ amino acids join together
77
New cards
Synthesis of dipeptides and polypeptides
2 amino acids joined by condensation reaction (between carboxyl group (OH) on one and amino group (H) on another) so a water molecule is lost
Covalent bond formed is a peptide bond
Covalent bond formed is a peptide bond
78
New cards
Breakdown of dipeptides and polypeptides
A water molecule is added during a hydrolysis reaction to break the peptide bond
79
New cards
Levels of protein structure
Primary
Secondary
Tertiary
Quaternary
Secondary
Tertiary
Quaternary
80
New cards
Protein primary structure
\-Sequence of amino acids in the polypeptide chain
\-Different proteins have different sequences of amino acids so a change in one may change the whole protein structure
\-Different proteins have different sequences of amino acids so a change in one may change the whole protein structure
81
New cards
Primary structure bonds
Peptide bonds
82
New cards
Protein secondary structure
\-Hydrogen bonds form between nearby amino acids in the polypeptide chain
\-They coil into an alpha helix or fold into a beta pleated sheet
\-They coil into an alpha helix or fold into a beta pleated sheet
83
New cards
Secondary structure bonds
Hydrogen bonds
84
New cards
Protein tertiary structure
\-The 3D shape of the polypeptide, when the secondary structure becomes even further coiled/folded
\-More bonds form between the R groups to stabilise it and hold it in place
\-More bonds form between the R groups to stabilise it and hold it in place
85
New cards
Tertiary structure bonds
\-Ionic bonds
\-Disulfide bonds
\-Hydrogen bonds
\-Hydrophobic and hydrophilic interactions
\-Disulfide bonds
\-Hydrogen bonds
\-Hydrophobic and hydrophilic interactions
86
New cards
Ionic bonds - tertiary structure
Attraction between positively charged R groups and negatively charged R groups on different amino acids
87
New cards
Disulfide bonds - tertiary structure
Covalent bonds between sulfur atoms of R groups of 2 cysteine amino acids
88
New cards
Hydrogen bonds - tertiary structure
Weak bonds between positively charged groups on one amino acid and negatively charged groups on another
89
New cards
Hydrophobic and hydrophilic interactions - tertiary structure
Hydrophobic R groups close together tend to clump and cluster on the inside of a protein
This means hydrophilic R groups are pushed to the outside to interact with surrounding water molecules
Effects how the protein folds up into its final structure
This means hydrophilic R groups are pushed to the outside to interact with surrounding water molecules
Effects how the protein folds up into its final structure
90
New cards
Protein quaternary structure
1+ polypeptide chains assembled together
Final 3D structure for proteins
Influenced by all bonds in previous structures
Determined by tertiary structures being bonded together
Final 3D structure for proteins
Influenced by all bonds in previous structures
Determined by tertiary structures being bonded together
91
New cards
Quaternary structure bonds
Peptide, hydrogen, ionic, disulfide bonds
92
New cards
Heating a protein up to a high temperature does what
Breaks up ionic and hydrogen bonds
Disrupts hydrophobic and hydrophilic interactions
Causes a change in proteins 3D shape
Disrupts hydrophobic and hydrophilic interactions
Causes a change in proteins 3D shape
93
New cards
Globular proteins
\-Circular shaped
\-Structural function
\-Soluble (easily transported in fluids)
\-Some are fairly reactive
\-Tertiary structure
\-Non polar hydrophobic R groups placed towards centre of protein
\-Polar hydrophilic R groups placed on outside of protein
\-eg. haemoglobin, amylase and insulin
\-Structural function
\-Soluble (easily transported in fluids)
\-Some are fairly reactive
\-Tertiary structure
\-Non polar hydrophobic R groups placed towards centre of protein
\-Polar hydrophilic R groups placed on outside of protein
\-eg. haemoglobin, amylase and insulin
94
New cards
Haemoglobin as an example of a globular protein with a prosthetic group
\-Carries oxygen around the body in red blood cells
\-The prosthetic group haem is attached, making haemoglobin a conjugated protein
\-Has a quaternary structure of 4 polypeptide chains (2 alpha, 2 beta)
\-The prosthetic group haem is attached, making haemoglobin a conjugated protein
\-Has a quaternary structure of 4 polypeptide chains (2 alpha, 2 beta)
95
New cards
Amylase as an example of a globular protein
\-An enzyme that catalyses the breakdown of starch
\-Made of a single chain of amino acids
\-Made of a single chain of amino acids
96
New cards
Insulin as an example of a globular protein
\-A hormone secreted by the pancreas
\-Regulates blood glucose level
\-Has 2 polypeptide chains held by disulfide bonds
\-Regulates blood glucose level
\-Has 2 polypeptide chains held by disulfide bonds
97
New cards
Fibrous proteins
\-Long stranded shape with cross linkages from hydrogen bonds
\-Physiological function
\-Insoluble
\-Fairly unreactive
\-Strong and flexible
\-Little tertiary structure
\-Large number of hydrophobic R groups
\-eg. collagen, keratin and elastin
\-Physiological function
\-Insoluble
\-Fairly unreactive
\-Strong and flexible
\-Little tertiary structure
\-Large number of hydrophobic R groups
\-eg. collagen, keratin and elastin
98
New cards
Collagen as an example of a fibrous protein
\-Very strong molecule in connective tissue (bone and cartilage)
\-Has Quaternary structure of 3 polypeptide chains
\-Has Quaternary structure of 3 polypeptide chains
99
New cards
Keratin as an example of a fibrous protein
\-Found in external structures
\-Flexible (skin)
\-Hard (nails)
\-Flexible (skin)
\-Hard (nails)
100
New cards
Elastin as an example of a fibrous protein
\-Stretches and returns to original shape
\-Found in elastic connective tissues (skin and tendons)
\-Found in elastic connective tissues (skin and tendons)