Basic Chemistry: Thermodynamics, Matter, and Measurement Concepts
1/121
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
122 Terms
First Law of Thermodynamics
Energy of the universe is conserved.
System
The area or location under study.
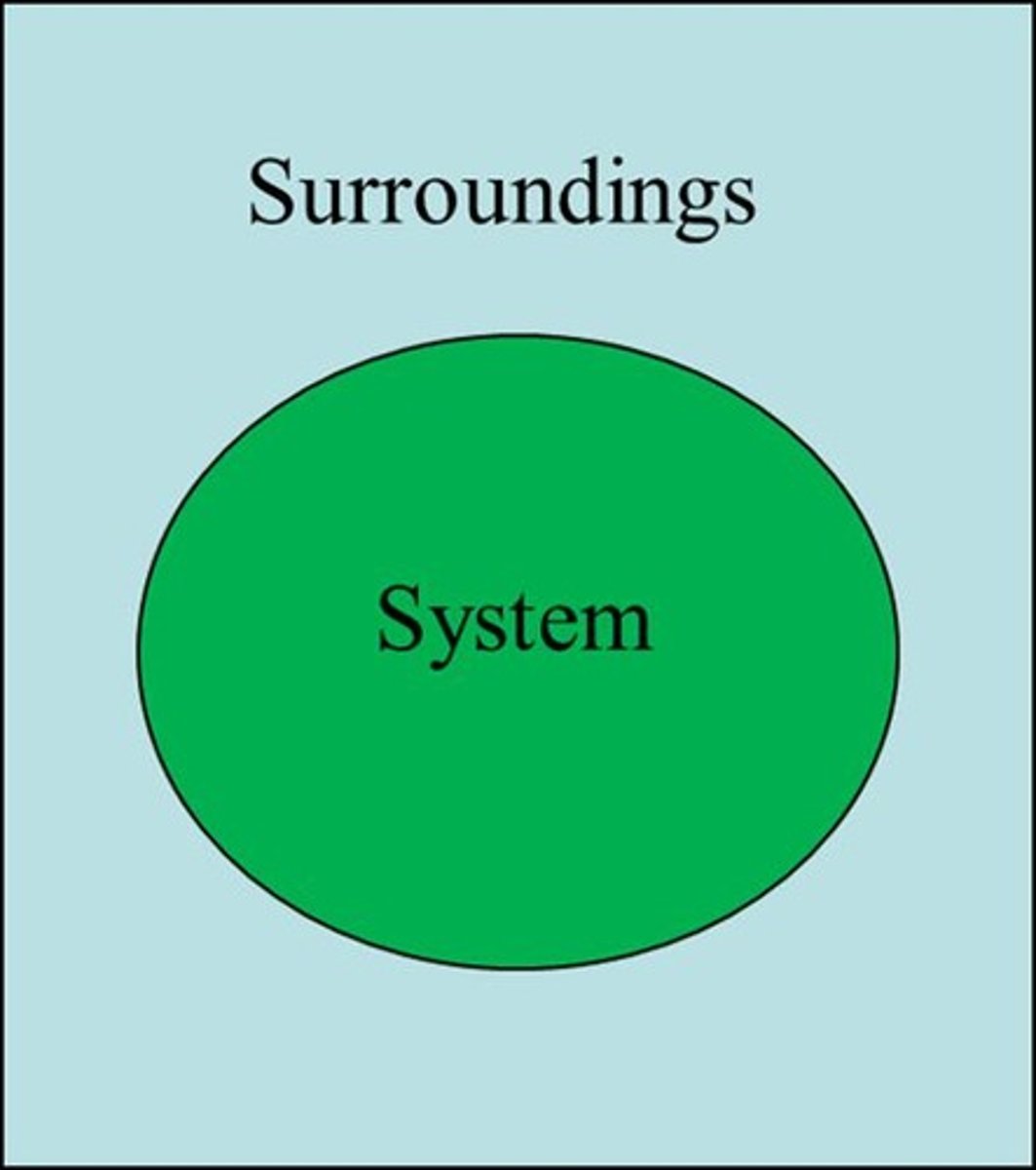
Surroundings
Area or location outside the system.
Universe
System + surroundings.
Exothermic
System loses heat to the surroundings; the energy of the system decreases whereas the energy of the surroundings increases.
Endothermic
System gains heat from surroundings; the energy of the system increases whereas the energy of the surroundings decreases.
Dimensional Analysis
Using units as a guide to solving problems.
Conversion Factor
A fractional quantity with the units we are converting from on the bottom and the units we are converting to on top.
Matter
Anything that has mass and occupies space (i.e., has volume).
Atoms
Basic submicroscopic particles that constitute the fundamental building blocks of ordinary matter.
Molecules
Particles formed when two or more atoms bond together in specific geometric arrangements.
Classification of Matter
Matter can be classified according to its state (solid, liquid, gas) and its composition or the types of particles.
Solid Matter
In solid matter, atoms or molecules pack closely to each other in fixed locations, resulting in a fixed volume and rigid shape.
Chemical Reaction
A process that leads to the transformation of one set of chemical substances to another.
Phase Change
A transition of matter from one state to another (e.g., solid to liquid).
Energy Flow
The transfer of energy from the system to the surroundings or vice versa.
Heat Sign Convention
Heat carries a negative sign in exothermic processes and a positive sign in endothermic processes.
Subatomic Particles
Particles such as neutrons, protons, and electrons that make up elemental atoms.
Physical Properties
Characteristics of matter that can be observed or measured without changing the substance.
Geometric Arrangements
The specific spatial arrangement of atoms in a molecule.
Increasing Temperature
The process that causes the state of matter to change from solid to liquid to gas.
Fixed Volume
A characteristic of solids where the volume does not change.
Rigid Shape
A characteristic of solids where the shape does not change.
Solid
A state of matter with a fixed volume and rigid shape.
Liquid Matter
Matter where atoms or molecules pack closely but can move relative to each other, having a fixed volume but not a fixed shape.
Gaseous Matter
Matter where atoms or molecules have a lot of space between them and are free to move relative to one another, making gases compressible.
Elements
Pure substances that cannot be chemically broken down into simpler substances, composed of a single type of atom.
Compounds
Substances composed of two or more elements in fixed, definite proportions.
Mixtures
Substances composed of two or more components in proportions that can vary from one sample to another.
Pure Substance
A substance made up of only one component with an invariant composition.
Heterogeneous Mixture
A mixture in which the composition varies from one region to another, made of multiple substances whose presence can be seen.
Homogeneous Mixture
A mixture that appears to be one substance, with all portions having the same composition and properties.
Scientific Method
A process for understanding nature based on observation and experimentation.
Observations
Descriptions about the characteristics or behavior of nature, also known as data.
Hypothesis
A tentative interpretation or explanation of observations.
Compressible
A property of gases that allows them to be compressed due to the space between atoms or molecules.
Water
A substance that is a liquid at room temperature.
Alcohol
A substance that is a liquid at room temperature.
Gasoline
A substance that is a liquid at room temperature.
Salt and Sand Mixture
An example of a heterogeneous mixture where the components can be seen.
Sweetened Tea
An example of a homogeneous mixture that appears to be one substance.
Basic Building Blocks of Matter
Elements that serve as the fundamental components of all matter.
Chemical Reactivity
The tendency of most elements to combine with other elements to form compounds.
Scientific Law
A brief statement that summarizes past observations and predicts future ones.
Law of Conservation of Mass
In a chemical reaction, matter is neither created nor destroyed.
Theory
A well-established hypothesis or set of hypotheses that explains why a natural phenomenon happens.
Dalton's Atomic Theory
A theory proposed by John Dalton that supported the early atomic ideas of Leucippus and Democritus.
Big Bang Theory
A scientific theory that explains the origin of the universe.
Qualifiable Data
Observational data that are subjective in nature, such as color and shape.
Quantifiable Data
Measurable (empirical) data that are objective in nature and require equipment to generate.
Empirical Data
Data obtained through observation and experimentation.
International System of Units (SI)
A standardized system of measurement used globally, also known as the Metric system.
Leucippus
A philosopher who, along with his student Democritus, proposed that matter was composed of small, indestructible particles.
Democritus
A philosopher who stated, 'Nothing exists except atoms and empty space; everything else is opinion.'
Plato
A philosopher who did not embrace the atomic ideas of Leucippus and Democritus.
Aristotle
A philosopher who held that matter had no smallest parts and proposed different substances were made of fire, air, earth, and water.
Law of Definite Proportions
A law stating that a chemical compound contains its component elements in fixed ratio by mass.
Law of Multiple Proportions
A law stating that when two elements combine to form more than one compound, the masses of one element that combine with a fixed mass of the other are in a ratio of small whole numbers.
Experimental Results
Outcomes of experiments that validate or invalidate a hypothesis or theory.
Falsifiable Hypothesis
A hypothesis that can be proven wrong through experimentation.
Scientific Measurement Importance
Scientific data can be either qualifiable or quantifiable, which is crucial for empirical research.
Standardized Units
Units of measurement that are consistent and accepted for scientific use.
Subjective Data
Data based on personal opinions, interpretations, feelings, and beliefs.
Objective Data
Data that is measurable and observable, not influenced by personal feelings.
Matter Composition
The idea proposed by early philosophers that matter is made up of small particles.
Copyright Notice
This work is protected by United States copyright laws and is intended for educational use only.
Qualitative observations
Descriptive in nature, such as changes in color and physical state.
Quantitative observations
Measurements that consist of numerical values obtained from instrumentation, glassware, and other measuring devices.
Counted values
Numerical values that represent counts, such as the number of cats per household in the United States.
Measurements
All measurements consist of two parts: a number that reflects the precision of the instrument and a unit.
Example of a measurement
25.0 centimeters or 1.00 feet.
Unit
A standard quantity used to specify measurements, which may be part of the International System of Units (SI) or the English system.
Standard Units of Measures (SI)
Length: meter (m), Mass: kilogram (kg), Time: second (s), Temperature: kelvin (K), Electric Current: ampere (A), Luminous Intensity: candela (cd).
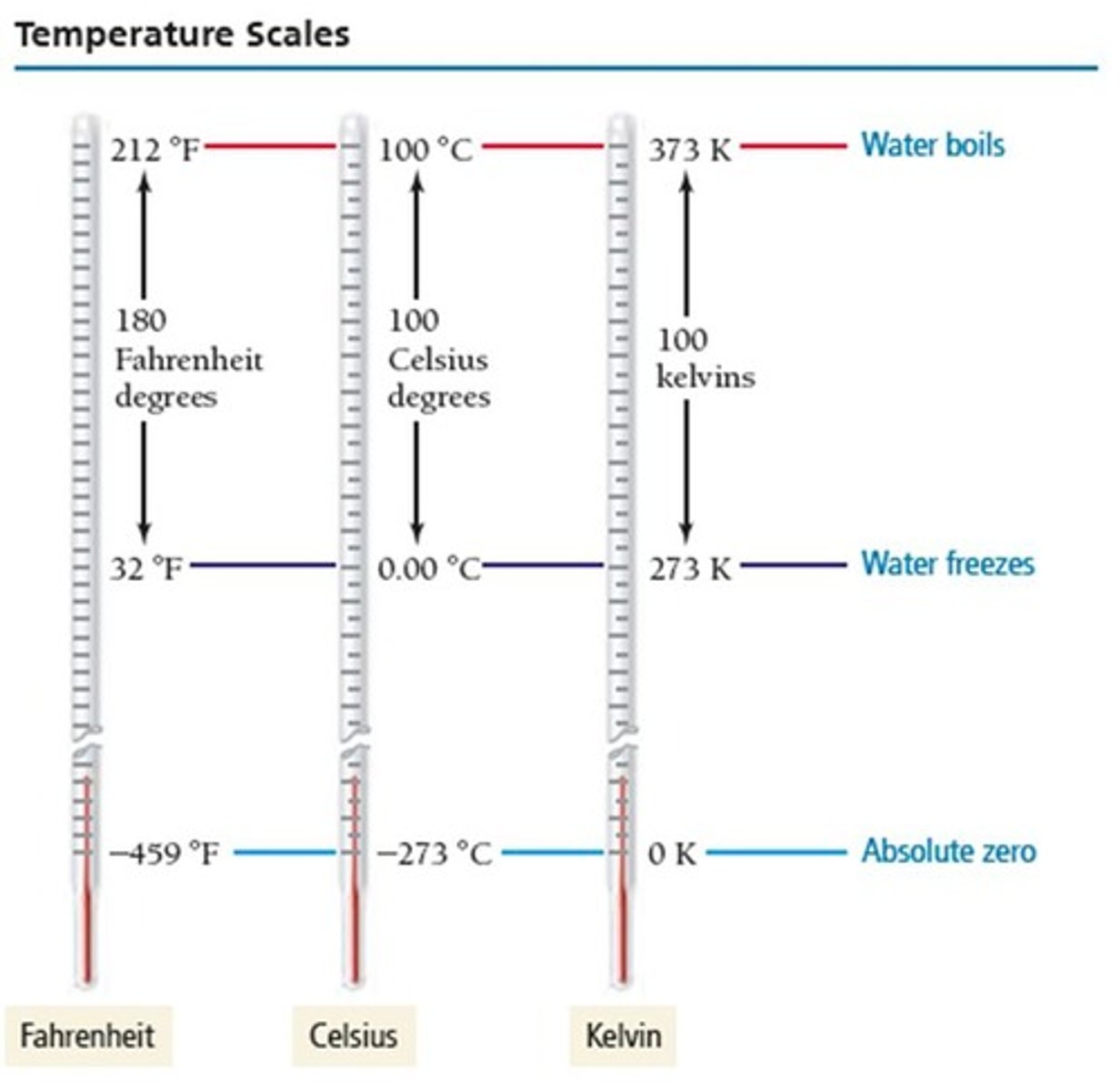
Temperature Calculation from Celsius to Kelvin
Use the equation: K = °C + 273.15.
Liquid nitrogen boiling point
Liquid nitrogen boils at 77 K.
Temperature scale with no negative temperatures
Kelvin.
Metric Prefix Multiplier
A prefix that indicates a factor of ten for a measurement, such as mega, milli, micro, or kilo.
Significant figures
The reporting of significant figures in an answer is dependent on the precision of the measured values or the precision of the glassware or laboratory equipment used.
Exact values
Measurements that have an infinite number of significant figures.
Example of exact value
1 inch = 2.54 centimeters.
Significant Figure Rule 1
All nonzero values are significant.
Significant Figure Rule 2
Zeroes between nonzero digits are significant.
Leading zeroes
Place-holder zeroes to the left of a nonzero digit are not significant.
Trailing zeroes
Zeroes to the right after a nonzero digit are not significant unless there is a decimal point.
Example of significant figures
536 has three significant figures.
Example of significant figures in scientific notation
140.00 has five significant figures.
Precision of Laboratory Glassware
Precision of the Last Digit is determined by estimating between the marks on a scale.
Measurement Reading Example
The graduated cylinder has markings every 0.1 milliLiters, and a reading of 4.56 milliLiters would be recorded.
Scientific Notation Example
3010 in scientific notation is 3.010 x 10^3.
Significant Figures in Measurement Problem
How many significant figures are in each number? a. 554 kilometers b. 7 pennies c. 0.00099 seconds d. 1.4500 kilometers e. 21,000 meters.
Mathematical Operations and Significant Figures
Mathematical operations dictate the reporting of significant figures in an answer.
Multiplication and Division Rule
The least precise measured value determines the number of significant figures in the reported answers.
Addition and Subtraction Rule
The value with the smallest decimal measurement determines the answer's significant figures.
Example of Reporting Significant Figures
Report only one significant figure using scientific notation to remove ambiguity.
Example of Reporting Length
Report 5 centimeters.
Example of Reporting Inches
You would report 336.68 inches.
Example of Reporting Centimeters
You would report 456 centimeters.
Density Definition
Density is defined as mass/volume.
Intensive Physical Property
Intensive physical properties are independent of the amount of substance being measured.
Extensive Physical Property
Extensive physical properties are dependent on the amount.