solids, liquids and gases
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
37 Terms
density is a measure of the compactness of a substance. relates the mass of a substance to how much space it takes up.
what is density?
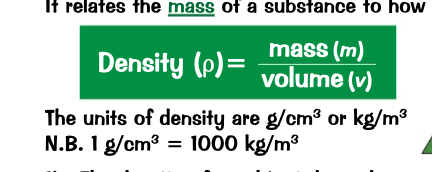
What is the formula for density?
what it is made out of. doesn’t vary with size or shape.
what does the density of object depend of?
whether it floats or sinks- a solid object will float on a fluid if it has a lower density than the fluid
what does the average density of an object determine?
1) to measure the density of a substance, use a balance to measure it’s mass.
2)if it’s a box shape start by measuring its length, width, and height with ruler. then calculate its volume by multiplying the length. width and height together.
3) for an irregular solid you can find its volume by submerging it in a eureka can filled with water. the water displaced by the object will be transferred to the measuring cylinder.
4) record the volume of water in the measuring cylinder.
use formula
practical to find density of an object from it’s mass and volume?
pressure is a measure of the force being applied to the surface of something.
relates to how much force is being applied to an object to the area that is applied over.
measure in pascals
what is pressure?

pressure formula
lower pressure
what will happen to the pressure is the same force is applied over a larger area?
pressure acts equally in all directions
in gases and liquids at rest what happens to pressure?
pressure increased with depth. pressure is higher at the bottom of the sea than at the surface and it is lower high up in the atmosphere
in gases and liquid what happens to pressure and depth?
density.
what does pressure difference in liquids and gases depend on?
the difference in pressure between two points in a liquid or gas. depends on the height difference and density of substance. gravity has an affect.
what is pressure difference?

pressure difference formula?
strong forces of attraction hold the particles close together in a fixed regular arrangement. dont have much energy so they can only vibrate about their fixed positions.
what happens in solids?
there are weaker forces of attraction between the particles. the particles are close together but can move past each other and form irregular arrangement. more energy than solid and move in random directions at low speeds.
what happens in liquids?
no forces of attraction between the particles. particles have most energy and free to move. move in random directions and at high speeds.
what happens in gases?
the extra energy is transferred into the particles kinetic energy store making them move faster. evenly when enough of the particles have enough energy to overcome their attraction to each other big bubbles of fas form in the liquid- boiling
what happens when you heat a liquid up?
the extra energy makes the particles vibrate faster until eventually the forces between them are partly overcome and the particles start to move around - melting
what happens when. you heat a solid?
1) fill a beaker with crushed ice. place a thermometer into the beaker and record the temperature of the ice.
2) using a bunsen, gradually heat the beaker full of ice
3) every 20 sec record the temperatures and the current state of the ice.
4) plot a graph of temperature against time.
experiment to show that temperature remains constant during changes of state:
a change of state at a constant temperature
what do the flat spots on a temperature time graph show?
as cold as stuff can get- 0 kelvins
what is absolute zero?
-273- absolute temperature have as little energy in their kinetic stores.
start of the Kelvin scale of temperature.
1 degrees one kelvin.
what is the coldest that anything can ever get?
+273
-273
how to convert from degrees to kelvin
kelvin to degrees
gases consist of very small particles which are constantly moving in completely random direction.
constantly collide and bounce off each other and the container walls
increase temp more energy
double temperature you double energy in the kinetic energy store of particles.
what doe the particle theory say?

what is directly proportional to what?
exert a force on it and their momentum and direction changes.
in. a sealed container gas particles smash against the container’s wall creating an outward pressure
pressure depends on how fast particles are going and how often they hit the wall
pressure and temp are proportional
what happens when gases collide with something?
inversely proportional
volume and pressure

constant temperature formula?/ fixed mass of gas at constant temperature

constant volume/ sealed container
temperature and energy
what does specific heat capacity relate to?
temperature
how can you measure the average internal energy of a substance?
warm up and also releases loads of energy when they could down.
what happens to materials that need to gain lots of energy?
energy required to change the temperature of an object by 1 degree per kilogram of mass.
what is the specific heat capacity of a substance?

what’s specific heat capacity of water?
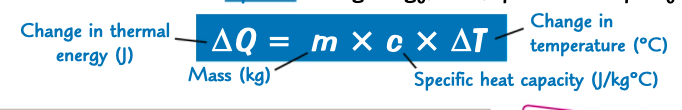
equation relating energy, mass, specific heat capacity and temperature
1) use a mass balance to measure the mass of the insulating container.
2) fill the container with water and measure the mass again. the difference in mass is the mass of the water in the container.
3) set up the experiment make sure the joule meter reads 0 and place a lid on the container
4) measure temp of water, then turn on power supply
5) keep an eye on thermometer. when temperature has increased by 10 switch of power and record the difference in increase and the energy of the joule meter
6) calculate using formula and repeat
how to find specific heat capacity of water?
1) make sure the block of material you use has 2 holes in it for the heater and thermometer and wrap it up with an insulating layer before starting. when you have switched off the power and finish timing wait until temp has stopped increased and record highest temp- gives energy from heater more time to spread through solid block.
how to find specific heat capacity of solid?