carboxylic acids and acyl chlorides and acid anhydrides
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
What is the suffix for carboxylic acids?
-oic acid
What 2 groups does a carboxylic acid have?
A carbonyl group and hydroxyl group
What shape do carboxylic acids have?
A planar shape, which cannot be rotated
What happens if the carboxylic group is in the middle of the carbon chain?
It becomes an ester
What is another name for ethanoic acid
Acetic acid
What is another name for benzene carboxylic acid
Benzoic acid
What is another name for methanoic acid
Formic acid
Describe the changes in the physical properties when the carbon chain of a carboxylic acid increases, and explain why
Boiling point increases because the IMFs overcome the Van der Waals forces
Dissolves more readily in Polar solvents because of more hydrogens available for hydrogen bonding
Describe the changes in the physical properties when the carbon chain of a carboxylic acid decreases, and explain why
Solubility decreases in Non-polar substances
Lower BP
Weaker IMFs
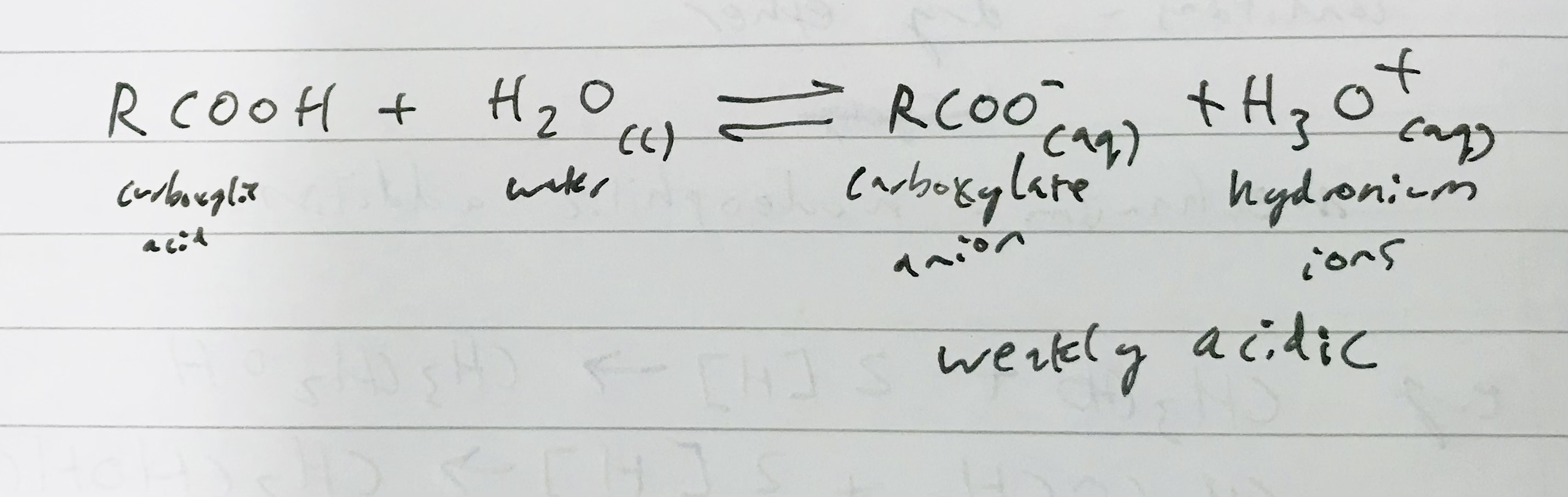
What happens when a carboxylic acid reacts with water?
It releases H+ ions when added to water, forming a weak acid
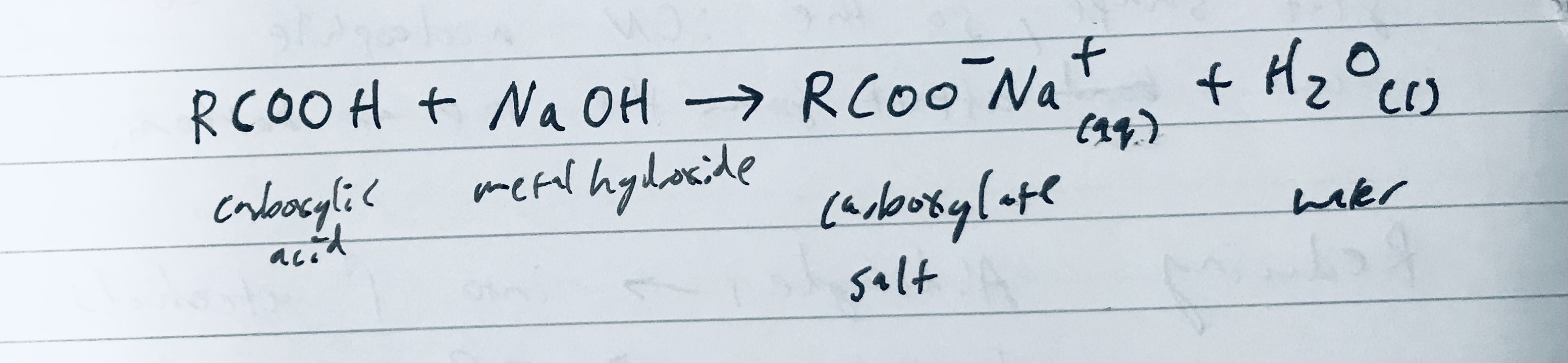
What happens when a carboxylic acid reacts with a hydroxide like sodium hydroxide?
It forms salts and water
Draw the symbol equation for the dissociation of a carboxylic acid in with water and give the products
Draw the symbol equation for the neutralization/deprotonisation of a carboxylic acid in with Sodium hydroxide and give the products
Draw the symbol equation for the acidification of a carboxylate salt reacting with Hydrochloric acid and give the products
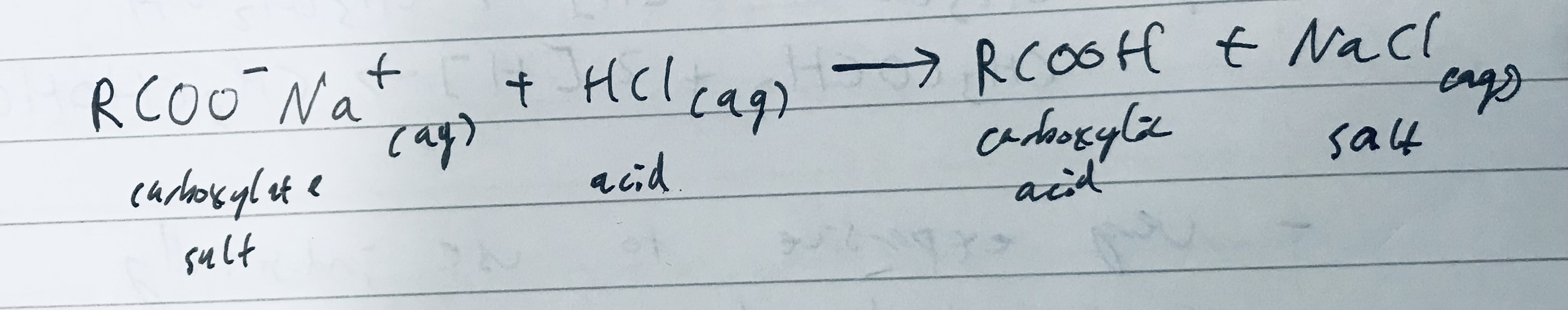
Draw the hydrogen bonding when methanoic acid reacts with one molecule of water.
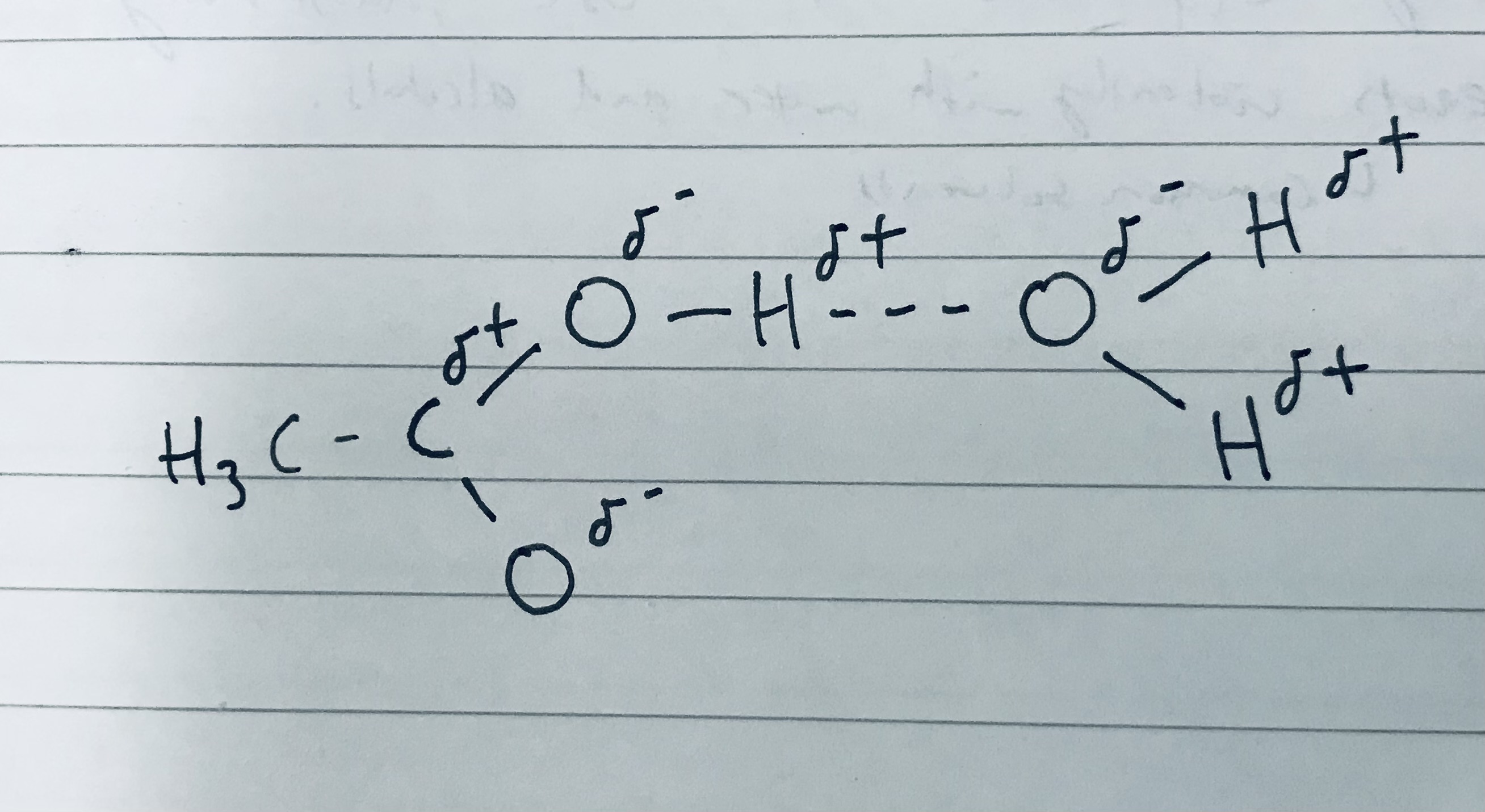
Give the molecular formula for ethanoic anyhydride when 2 moles of methanoic acid are hydrated
(CH3CO)2O
Give the molecular formula for ethanoyl chloride
CH3COCl
Give for examples of nucleophiles in addition elimination reactions
Water, alcohol, ammonia and amines
Give the reaction mechanism of ethanoyl chloride reacting with water, and give its constituent product/s
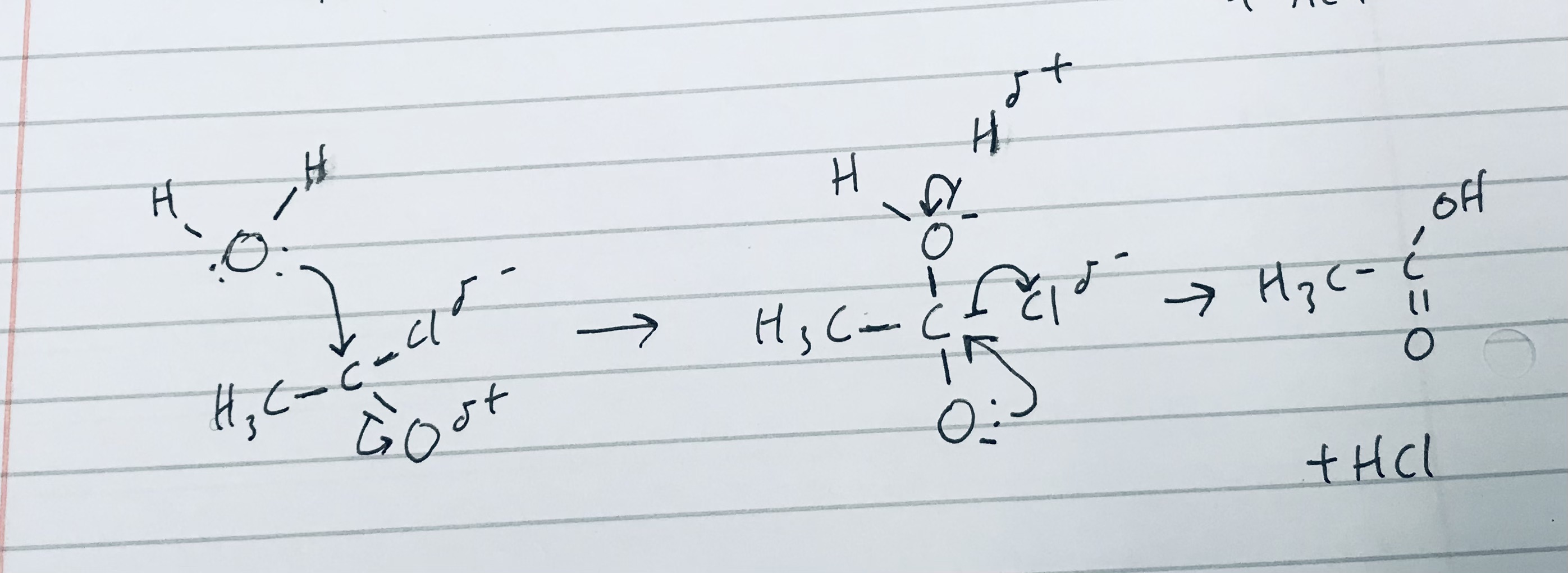
Give the reaction mechanism of ethanoyl chloride reacting with methanol, and give its constituent product/s
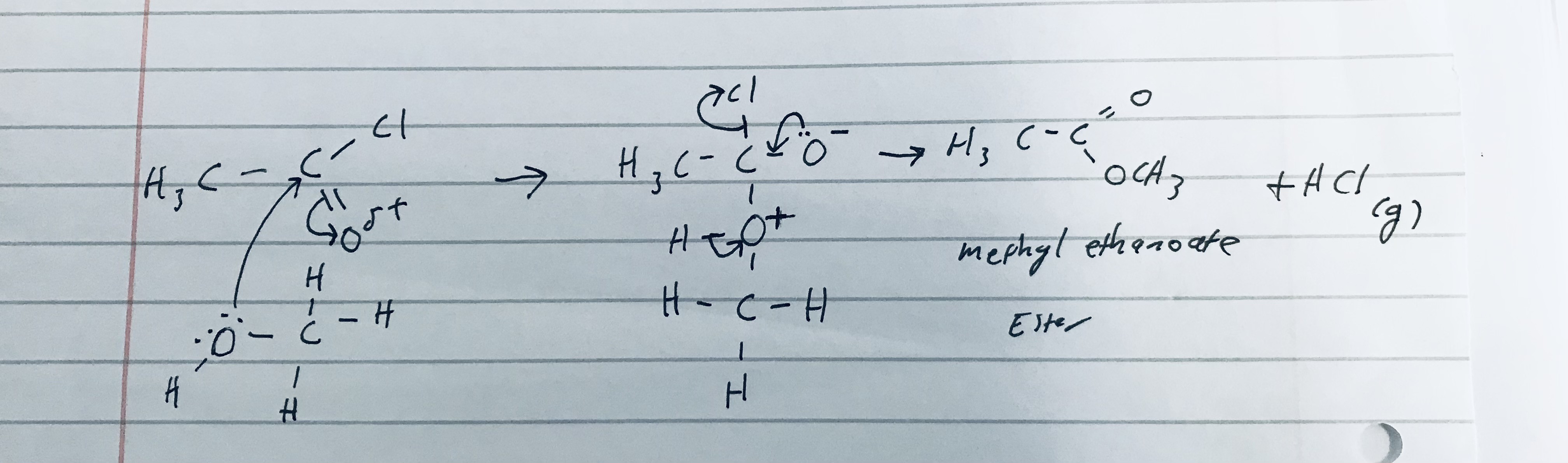
Give the reaction mechanism of ethanoyl chloride reacting with ammonia, and give its constituent product/s
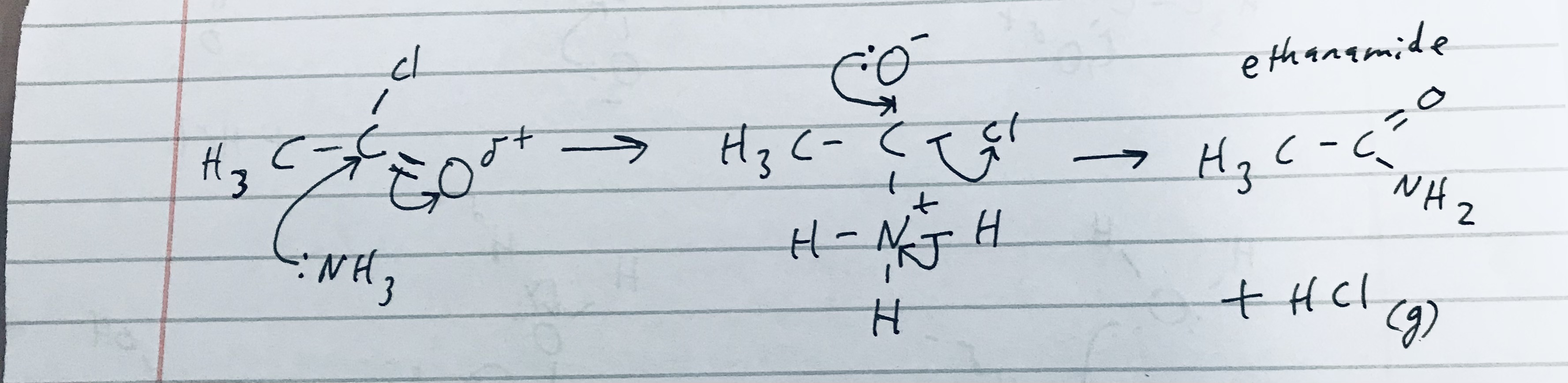
Give the reaction mechanism of ethanoyl chloride reacting with Ethanamine (primary amine), and give its constituent product/s
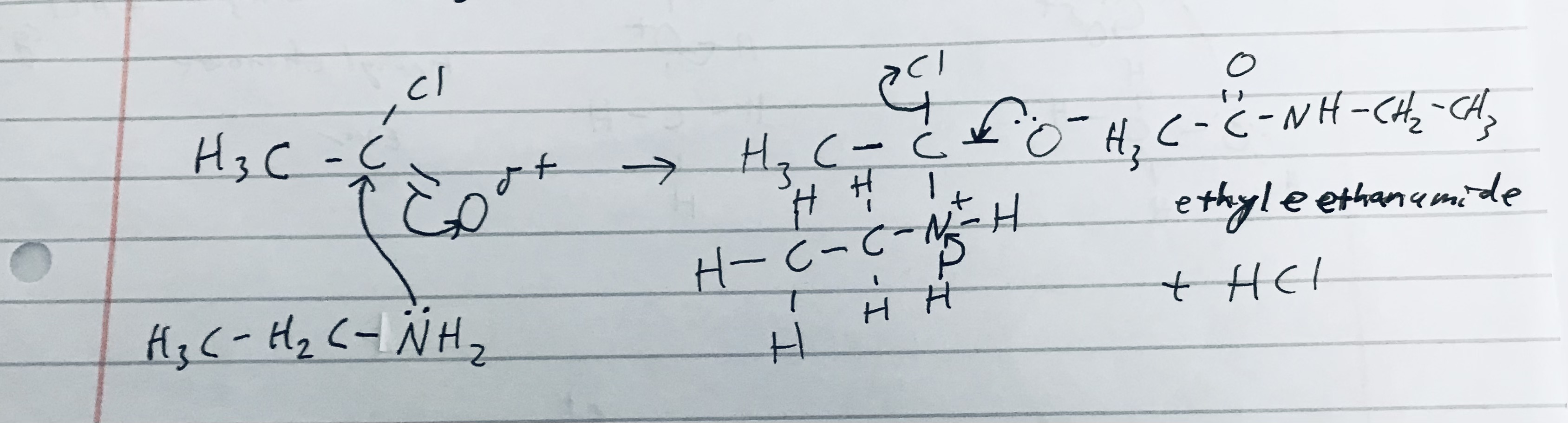
Why are acid anhydrides better than using carboxylic acids
Acid anhydrides are not as reactive as acyl chlorides so the
reaction is slower.
The reaction is safer - it is less exothermic.
It is less corrosive and not so readily hydrolysed (its reaction
with water is slower).
Acid anhydrides are less toxic. Ethanoic anhydride doesn't
produce dangerous (corrosive and poisonous) fumes of
hydrogen chloride.
Ethanoic anhydride is cheaper than ethanoyl chloride.
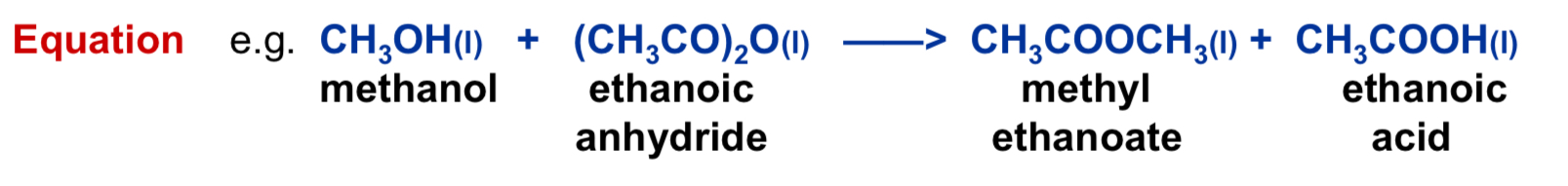
Give the chemical equation and word equation of methanol reacting with ethanoic anyhydride