Section 1 - Biological molecules
1/137
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
138 Terms
What is covalent bonding
The sharing of electrons between two non-metals.
The outer shell of both atoms is filled and a more stable compound called a molecule is formed.
What is ionic bonding
The transfer of electrons from a metal to a non-metal.
Metals will form positive ions.
Non- metals will form negative ions.
Between oppositely charged ions there will be an electrostatic attraction.
What is hydrogen bonding
It’s weak attraction between opposite dipoles.
In some covalent bonds, electrons are not shared evenly, so one atom becomes slightly negative and the other slightly positive.
This makes the molecule polar.
A hydrogen bond forms when the slightly positive atom of one molecule is attracted to the slightly negative atom of another molecule.
A weak electrostatic bond is formed between the two.
Although each bond is individually weak, they can collectively form important forces that alter the physical properties of molecules.
The element that contains one proton and one electron
Hydrogen
Monomer definition
The single sub-units, or building blocks, of life.
Examples of monomers
Amino acids
Nucleotides
Monosaccharides
Polymer definition
Large molecule made up of many repeating monomers joined together by covalent bonds.
Polymer examples
Starch
DNA
Protein
What is polymerisation
Monomers can be linked together by covalent bonds to form long chains.
These long chains are called polymers and the process by which they are formed is polymerisation.
What are macromolecules
Very large molecules
Have high molecular mass
Macromolecule examples
Proteins
Nucleic acids
Polysaccharides
What is a condensation reaction
Reaction where two molecules are joined together by covalent bonds and a water molecule is released.
In polymerisation, each time a monomer is added a water molecule is formed.
Products of condensation
Amino acids → protein
Two monosaccharides→ disaccharide
Fatty acids + monoglycerides→ lipids
What is a hydrolysis reaction
Reaction in which a covalent bond is broken by the addition of water, splitting a polymer into monomers.
Products of hydrolysis
Proteins → amino acids
Carbohydrates → disaccharides and monosaccharides
Lipids → fatty acids + monoglycerides
Metabolism definition
All the chemical processes that take place in living organisms.
Anabolic and catabolic reactions.
What is a mole
The amount of substance that has the same number of particles as the number of carbon-12 atoms in 12g.
One mole is 6.02 × 10²³ particles (the Avogadro constant).
What is a molar solution
A solution that contains 1 mole of solute in each litre of solution.
Why is carbon able to form such a wide variety of organic molecules?
Carbon atoms can form 4 covalent bonds, which causes them to readily form bonds with other carbon atoms.
This allows a sequence of carbon atoms of various lengths to be built up.
These form a backbone along which other atoms can be attached.
This allows a large number of different types and sizes of molecule, all based on carbon.
What are carbon-containing molecules called?
Organic molecule.
What are carbohydrates
Carbon molecules combined with water.
They are made from monosaccharides.
Main types of carbohydrates
Monosaccharides
Disaccharides
Polysaccharides
What are the elements present in carbohydrates?
Carbon (C)
Hydrogen (H)
Oxygen (O)
Monosaccharide definition
The simplest carbohydrates, consisting of single sugar monomers that can’t be hydrolysed further.
What is the general formula of a monosaccharide?
(CH2O)n
Examples of monosaccharides
Glucose
Galactose
Fructose
Disaccharide definition
A carbohydrate made of two monosaccharides joined together by a glycosidic bond in a condensation reaction.
Disaccharide examples
Maltose = glucose + glucose
Sucrose = glucose + fructose
Lactose = glucose + galactose
Polysaccharide definition
A carbohydrate made of many monosaccharides joined by glycosidic bonds through a condensation reactions, forming a polymer.
Examples of polysaccharides
Starch
Glycogen
Cellulose
Chitin
What is a glycosidic bond
A covalent bond formed between monosaccharides in a condensation reaction, releasing a molecule of water.
What is the function of carbohydrates?
Energy storage (starch, glycogen)
Energy source (glucose)
Structural support (cellulose,chitin)
Starch structure
Starch is a polysaccharide
Monomer: a-glucose
Bond type: glycosidic bonds
Two types of chains:
Amylose - (unbranched, forms a coiled helix) → a-1,4 glycosidic bonds
amylopectin (branched) → a-1,6 glycosidic bonds.
Only found in plant cells.
Starch function
Starch is used as an energy storage molecule.
Insoluble - it doesn’t affect water potential, so water is not drawn into cells by osmosis.
Large - cannot diffuse out of cells, so storage is stable.
Coiled/compact structure - a lot can be stored in a small space.
Branched - has many ends, so enzymes can act on them simultaneously which creates a rapid release of glucose monomers which are easily transported and used in respiration.
Glycogen structure
Glycogen is a polysaccharide
Monomer: a glucose
Bond type: glycosidic
a-1,4 glycosidic bonds in the main chain
a-1,6 glycosidic bonds at branch points
Highly branched
Found in animal cells - in liver cells, muscle cells and bacteria.
Glycogen function
Glycogen is used for energy storage in animals.
Insoluble - it doesn’t affect water potential so water is not drawn into the cells by osmosis.
Large - it does not diffuse out of cells, so storage is stable.
Compact - a lot can be stored in small spaces.
Highly branched - has a lot of ends that can be acted on simultaneously by enzymes. It’s therefore more rapidly broken down to form glucose monomers which are used in respiration. This is important to animals which have a higher metabolic rate and therefore respiratory rate than plants because they are more active.
Structure of cellulose
Cellulose is a polysaccharide
Monomer: B-glucose
Bond type: B-1,4 glycosidic
Straight, unbranched chains
These chains run parallel to each other and are cross linked by hydrogen bonds.
These chains group together to form microfibrils.
Found in plant cell walls.
Function of cellulose
Provides structural support in plant cell walls.
Maintains cell shape and prevents bursting from osmotic pressure.
Strong and rigid due to hydrogen bonding between chains.
What is glucose
A hexose (6 carbon sugar) monosaccharide.
Has the chemical formula C6H12O6.
An important source of energy in humans.
What are the two types of glucose?
a-glucose and B-glucose.
They are isomers. They have the same molecular formula (C6H12O6) but a different arrangement of atoms in space.
The carbon atoms are numbered from 1-6 and the OH (hydroxyl) group are in a different orientation around C1.
What is a reducing sugar
A sugar that can donate electrons to another chemical (Benedict’s reagent).
All monosaccharides are reducing sugars.
Some disaccharides are reducing sugars.
What is Benedict’s Reagent
It’s an alkaline solution of copper (II) sulfate.
When a reducing sugar is heated with Benedict’s reagent it forms an insoluble red precipitate of copper (I) oxide.
Explain why Benedict’s Reagent turns red when heated with a reducing sugar.
The sugar donates electrons that reduce blue copper (II) sulfate to red copper (I) oxide.
Suggest a way, other than comparing colour changes, in which different concentrations of reducing sugar could be estimated.
Dry the precipitate in each sample and weigh it. The heavier the precipitate the more reducing sugar is present.
Explain why it’s not possible to distinguish between very concentrated samples of Benedict’s reagent, even when their concentrations are different.
Once all the copper (II) sulfate has been reduced to copper (I) oxide, further amounts of reducing sugar cannot make a difference.
Test for reducing sugars (Benedict’s test)
Add 2cm³ of the food sample to a test tube.
Add equal volume of Benedict’s reagent.
Heat the mixture in a gently boiling water bath for five minutes.
Positive - brick red.
Negative - no colour change (blue).
Test for non-reducing sugars
2cm³ of food sample/sugar solution.
2cm³ of dilute hydrochloric acid.
Boil for five minutes.
2cm³ of sodium hydrogen carbonate.
Then do Benedict’s test.
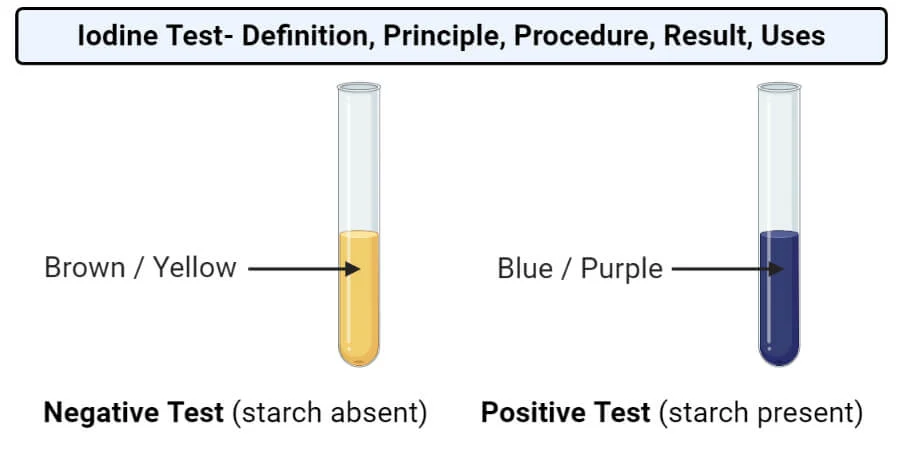
Iodine test for starch
Place 2cm³ of the sample being tested into a test tube.
Add two drops of iodine solution and shake or stir.
Positive - blue/black.
Lipid solubility
Insoluble in water.
Soluble in organic solvents - alcohol.
What are the two types of lipids
Triglycerides (fats and oils)
Phospholipids
Function of lipids
Source of energy - When oxidised, lipids provide more than twice the energy as the same mass of carbohydrate.
Waterproofing - Insoluble in water. Plants and insects have waxy, lipid cuticles that conserve water. Mammals produce an oily secretion from the sebaceous glands in the skin.
Insulation - Fats are slow conductors of heat and when stored beneath the body surface help to retain body heat. They also act as electrical insulators in the myelin sheath around nerve cells.
Protection - Fat is often stored around delicate organs, such as the kidney.
Organisms that move use lipids rather than carbohydrates as an energy store. Explain why this is.
When oxidised, lipids provide twice the energy as the same mass of carbohydrate.
If fat is stored, the same amount of energy can be provided for less than half the mass.
It’s a lighter storage product → major advantage for mobile organisms.
What are lipids made of?
Carbon (C)
Hydrogen (H)
Small amount of Oxygen (O)
Test for lipids
Mix test solution with ethanol.
Shake for around 1 minute.
Add water.
Positive - cloudy solution.
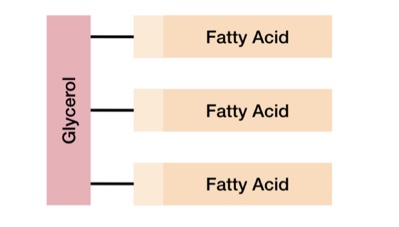
What is a triglyceride
A type of lipid made of one glycerol molecule and three fatty acids.
Each fatty acid forms an ester bond with glycerol in a condensation reaction.
There are over 70 different fatty acids which leads to a variation in the structures of triglycerides.
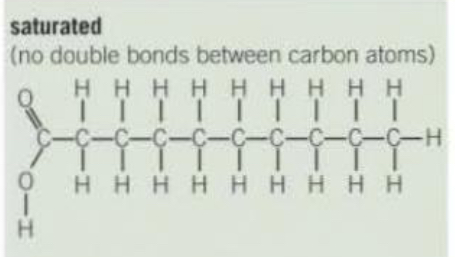
Saturated triglyceride structure
No carbon-carbon double bonds.
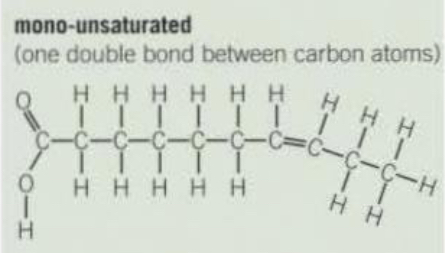
Mono-saturated/unsaturated triglyceride structure
One double bond between carbon atoms.
Polyunsaturated triglyceride structure
More than one double bond between carbon atoms.
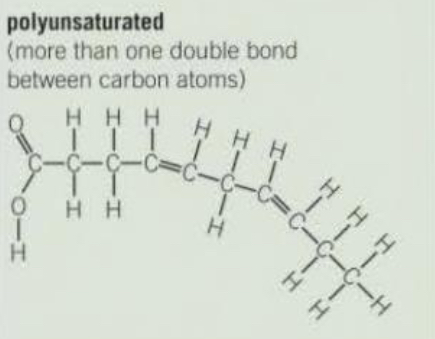
Why are unsaturated fatty acids typically liquid at room temperature?
The double bonds cause the molecule to bend, preventing the fatty acids from packing closely together, so they remain liquid (oils) at room temperature.
Functions of triglycerides
Source of energy - high ratio of energy-storing carbon-hydrogen bonds to carbon atoms.
Good storage - low mass to energy ratio → a large amount of energy can be stored in a small volume. This is especially beneficial to animals as it reduces the mass they have to carry around.
Insoluble in water - their storage doesn’t affect osmosis or the water potential of cells.
Important source of water - they have a high ratio of hydrogen to oxygen atoms, so when they are oxidised, they release water and provide a source of water. This is especially beneficial for organisms living in deserts.
What are phospholipids
A type of lipid that has a phosphate group, a glycerol molecule and two fatty acids.
The fatty aids are joined with an ester bond.
The phosphate head is hydrophilic.
The fatty acid tail is hydrophobic.
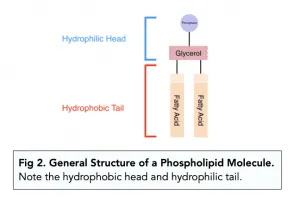
Functions of phospholipids
Phospholipids form a bilayer within cell-surface membranes, with hydrophilic heads facing outwards and hydrophobic tails inward.
A hydrophobic barrier is formed between the inside and outside of a cell.
Controls the movement of substances in and out of a cell.
What are amino acids
Amino acids are the monomers which combine to make up a polymer called a polypeptide.
Polypeptides can be combined to form proteins.
There are 20 different types of amino acids that are common in all organisms - they differ only in their side (R) group.
What are the amino acids four chemical groups?
Amino group - a basic group which gives the amino acid the amino part of its name.
Carboxyl group - an acidic group which gives the amino acid the acid part of its name.
Hydrogen atom
R (side) group - a range of chemical groups. Each amino acid has a different R group.
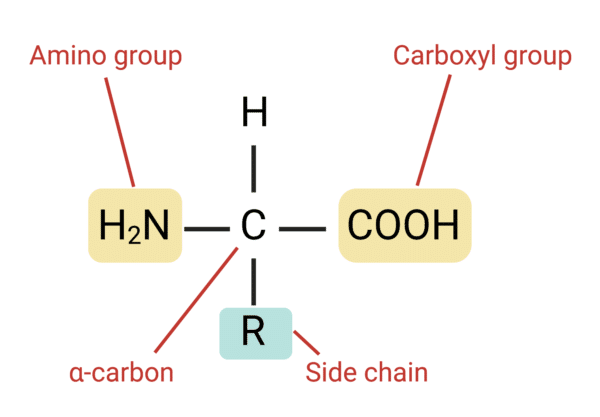
What is a peptide bond
A covalent bond between two amino acids via a condensation reaction forms a peptide bond.
The water is made by combining an -OH from the carboxyl group of one amino acid with an -H from the amino group of another amino acid.
The two amino acids then become linked by a new peptide bond between the carbon atom of one amino acid and the nitrogen atom of the other.
What is a dipeptide
Two amino acids joined together by a peptide bond through a condensation reaction.
What is a polypeptide
A polymer made of many amino acids joined together by peptide bonds in condensation reactions.
Examples of proteins
Enzymes
Structural proteins
Antibodies
Transport proteins
What is the structure of structural proteins
Long, strong polypeptide chains.
Structural proteins are connected by cross-links that hold the chains parallel to each other.
Examples of structural proteins
Collagen
Keratin
What are antibodies
Made up of polypeptide chains.
Type of globular proteins.
Used in the immune response.
Antibodies are diverse proteins.
Each antibody has a different sequence of amino acids.
What is the function of transport proteins
Transport proteins include channel proteins.
Transport molecules across the cell membrane.
Contain hydrophobic and hydrophilic amino acids.
Examples of transport proteins
Haemoglobin → carries oxygen in red blood cells.
Myoglobin → stores oxygen in muscles.
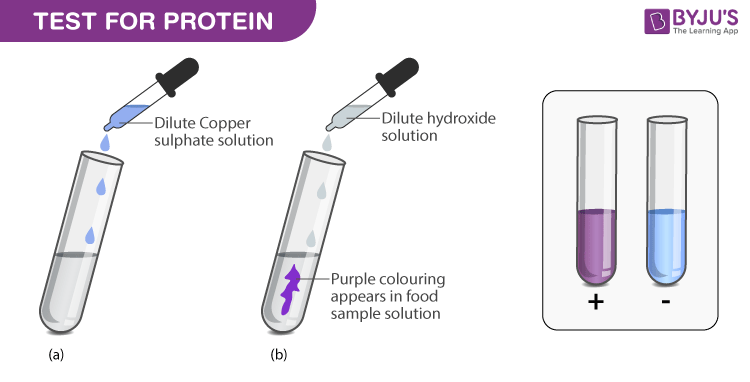
Biuret tests for proteins
Detects peptide bonds.
Equal volumes of test solution and sodium hydroxide.
Add few drops of dilute copper (II) sulfate.
Positive - solution will turn purple.
Order of protein structure
Amino acids → primary structure → secondary structure→ tertiary structure → quarternary structure
Explain the primary structure of proteins
The primary structure → the sequence of amino acids in a polypeptide chain.
Amino acids are linked by peptide bonds via condensation reactions.
This sequence is determined by the DNA base sequence.
The order of amino acids determines how the protein will fold into secondary and tertiary structures, and thus determines its function.
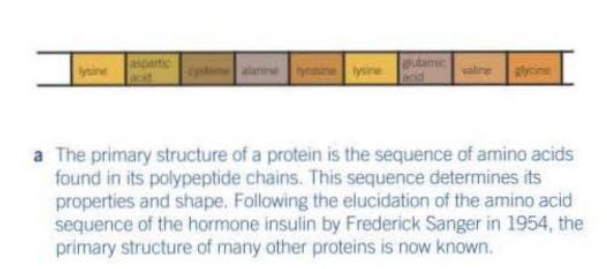
Explain the secondary structure of a protein
Weak hydrogen bonds form between the backbone of the polypeptide chains making it coil into an a-helix or a B-pleated sheet.
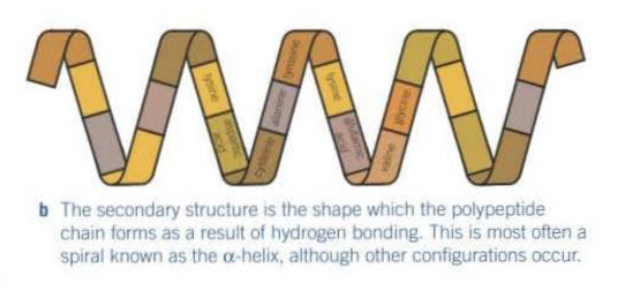
Explain the tertiary structure of a protein
Tertiary structure → the overall 3D shape of a single polypeptide chain.
It’s formed by interactions between the R-groups of the amino acids.
These interactions include:
Disulfide bridges - very strong, not easily broken
Hydrogen bonds - weak, numerous but easily broken
Ionic bonds - formed between any carboxyl and amino groups that aren’t involved in forming peptide bonds. They are broken by changes in pH.
The tertiary structure determines the proteins specific shape and function.
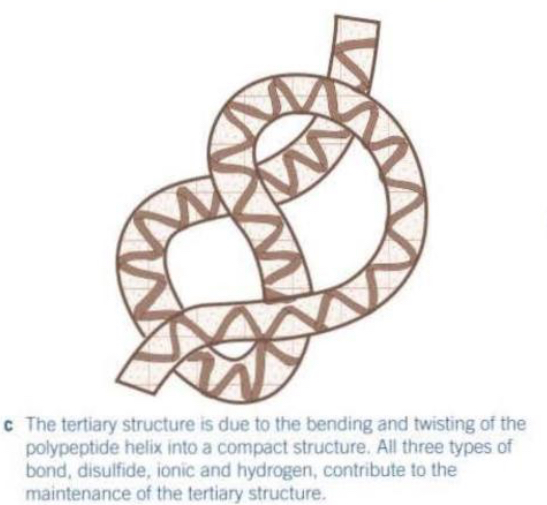
Explain the quarternary structure of a protein
For larger complex proteins more than one polypeptide chain may be involved.
The chains are held together by disulfide bridges, hydrogen bonds and ionic bonds.
Examples:
Fibrous proteins such as collagen which have a structural function.
Globular proteins such as enzymes, haemoglobin and antibodies carry out metabolic functions.
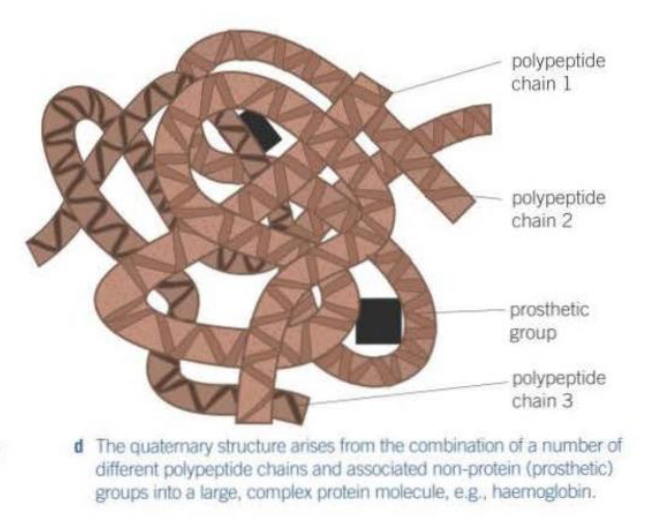
Explain the structure of fibrous proteins
Made from long polypeptide chains that run parallel to each other.
The chains are linked by cross-bridges.
Collagen structure
Primary structure → unbranched polypeptide chain.
Secondary structure → the polypeptide chain is very tightly wound.
Lots of the amino acid, glycine helps close packing.
Tertiary structure → the chain is twisted into a second helix.
Quaternary structure → made up of three such polypeptide chains wound together like a rope.
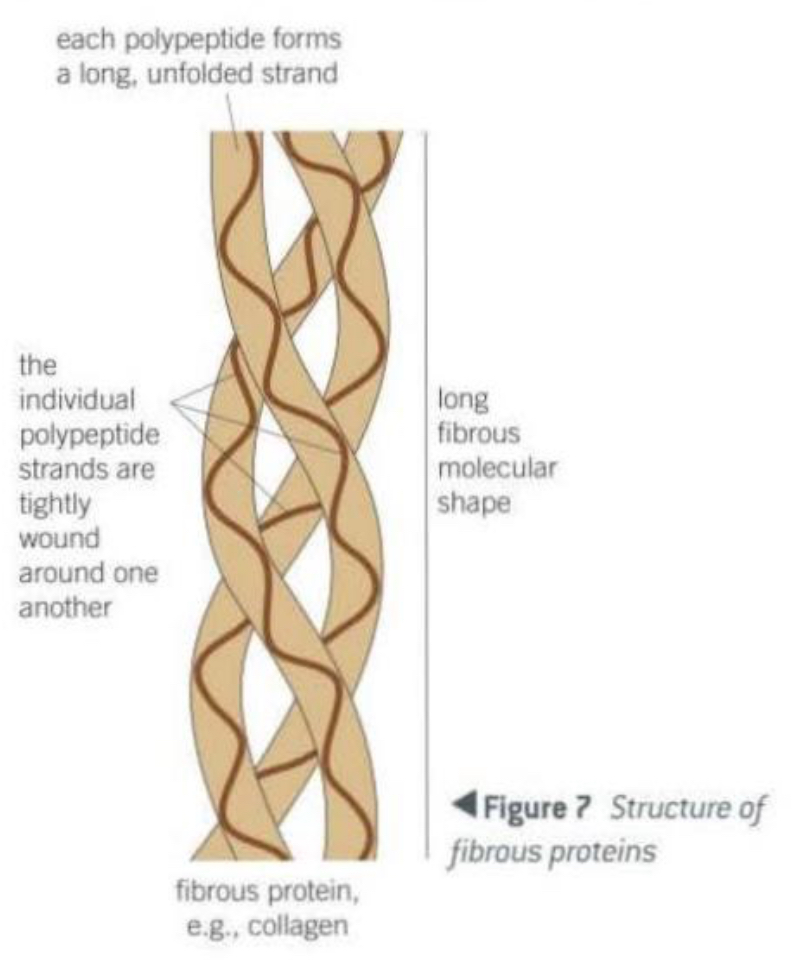
Where is collagen found and what is its function in the body?
Found in tendons.
Tendons join muscles to bones.
When a muscle contracts the bone is pulled in the direction of the contraction.
Explain how the cross-linkages between the amino acids of polypeptide chains increase the strength and stability of a collagen fibre.
It prevents the individual polypeptide chains from sliding past one another and so they gain strength because they act as a single unit.
The points where one collagen molecule ends and the next begins are spread throughout the fibre rather than all being in the same position along it.
Explain why this arrangement of collagen molecules is necessary for the efficient functioning of a tendon.
If all the ends of collagen molecules were aligned in the same position, the tendon would have a single weak point that could tear under tension.
By staggering the ends of the collagen molecules, the weak points are distributed.
This arrangement spreads the stress when the tendon is stretched, making it stronger and more resistant to tearing.
What are enzymes
Enzymes are globular proteins that act as biological catalysts.
They lower the activation energy required for a reaction to take place.
They don’t get used up so they can catalyse a large number of reactions.
Only a small amount of enzyme is needed to convert a large amount of substrate into product efficiently.
What is activation energy
Many reactions require an initial amount of energy to start.
The minimum amount of energy needed to activate the reaction in this way is called the activation energy.
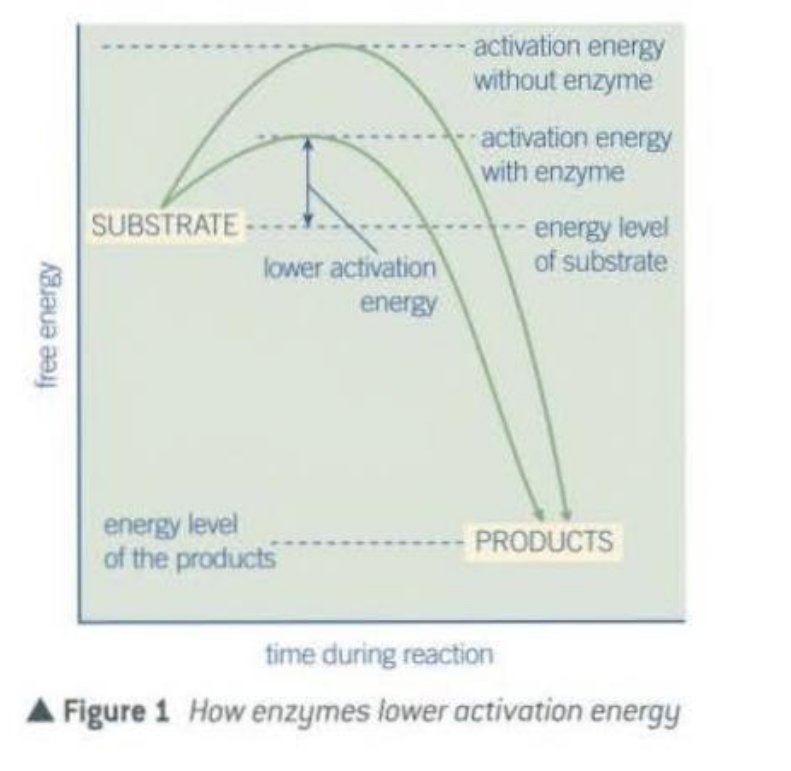
Why are enzymes specific to their substrate
The enzymes active site is complementary to only one substrate.
The substrate has a specific and complementary shape to the active site which allows it to bind and form an enzyme-substrate complex.
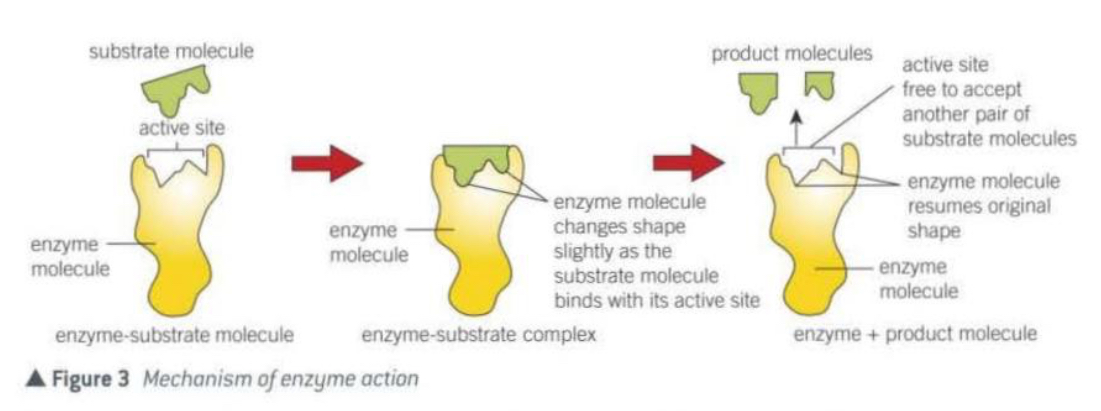
Explain the lock and key model for enzymes
There needs to be an exact match between the substrate and the active site.
Only then, will the enzyme-substrate complex be formed.
Each key has a specific shape that fits and operates only a single lock.
The lock is the enzyme.
The key is the substrate.
Limitation of a lock and key model for enzymes
The model assumes that the enzymes active site is rigid and the substrate fits perfectly without the enzyme changing shape.
Explain the enzyme induced fit model
The substrate approaches the enzymes active site.
As the substrate begins to bind, the enzyme slightly changes shape of its active site to better fit the substrate.
The enzyme is flexible.
With the substrate now in its right orientation, the enzyme can catalyse the reaction.
Then, the products are released and the enzyme returns to its original shape.
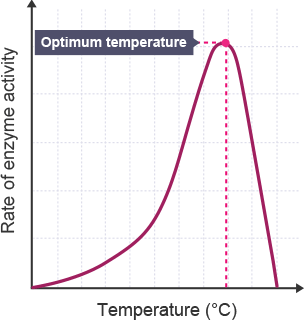
Affect of temperature on enzymes
As temperature increases, particles will move more and there will be more collisions so the enzyme is more likely to meet the substrate and form the enzyme substrate complex.
As temperature keeps rising the bonding within the enzyme starts to break down.
This causes a change in the shape of the active site so it’s no longer complementary to the substrate.
No enzyme-substrate complexes can form so the rate of reaction decreases. The enzyme has denatured.
Affect of pH on enzymes
The hydrogen ions [H+] and hydroxide ions [OH-] interfere with the ionic bonds in the tertiary structure. This changes the shape of the active site.
Enzymes only work within a certain range of pH, too acidic or too alkali, then it won’t work - the enzyme will be denatured.
Different enzymes work at different optimal pHs.
![<ul><li><p>The hydrogen ions [H+] and hydroxide ions [OH-] interfere with the ionic bonds in the tertiary structure. This changes the shape of the active site.</p></li><li><p>Enzymes only work within a certain range of pH, too acidic or too alkali, then it won’t work - the enzyme will be denatured.</p></li><li><p>Different enzymes work at different optimal pHs.</p></li></ul>](https://knowt-user-attachments.s3.amazonaws.com/0b78796c-b6c9-4e88-b1ec-a2aa5d88026b.png)
What is pH
The concentration of hydrogen ions [H+].
pH 1 is acidic with a high concentration of hydrogen ions.
pH 7 is neutral, with a balanced number of hydroxide ions [OH-] and hydrogen ions.
pH 14 is alkaline with high hydroxide ions, low hydrogen ions.
Affect of enzyme concentration on enzymes
A - Too few enzyme molecules to allow all substrate molecules to find an active site at one time. The rate of reaction is only half the maximum possible.
B - All the active sites are occupied at the same time. The rate of reaction has doubled to its maximum, because all active sites are filled.
C - The addition of further enzyme molecules has no effect as all active sites are already occupied at one time. There is no change in the rate of reaction.
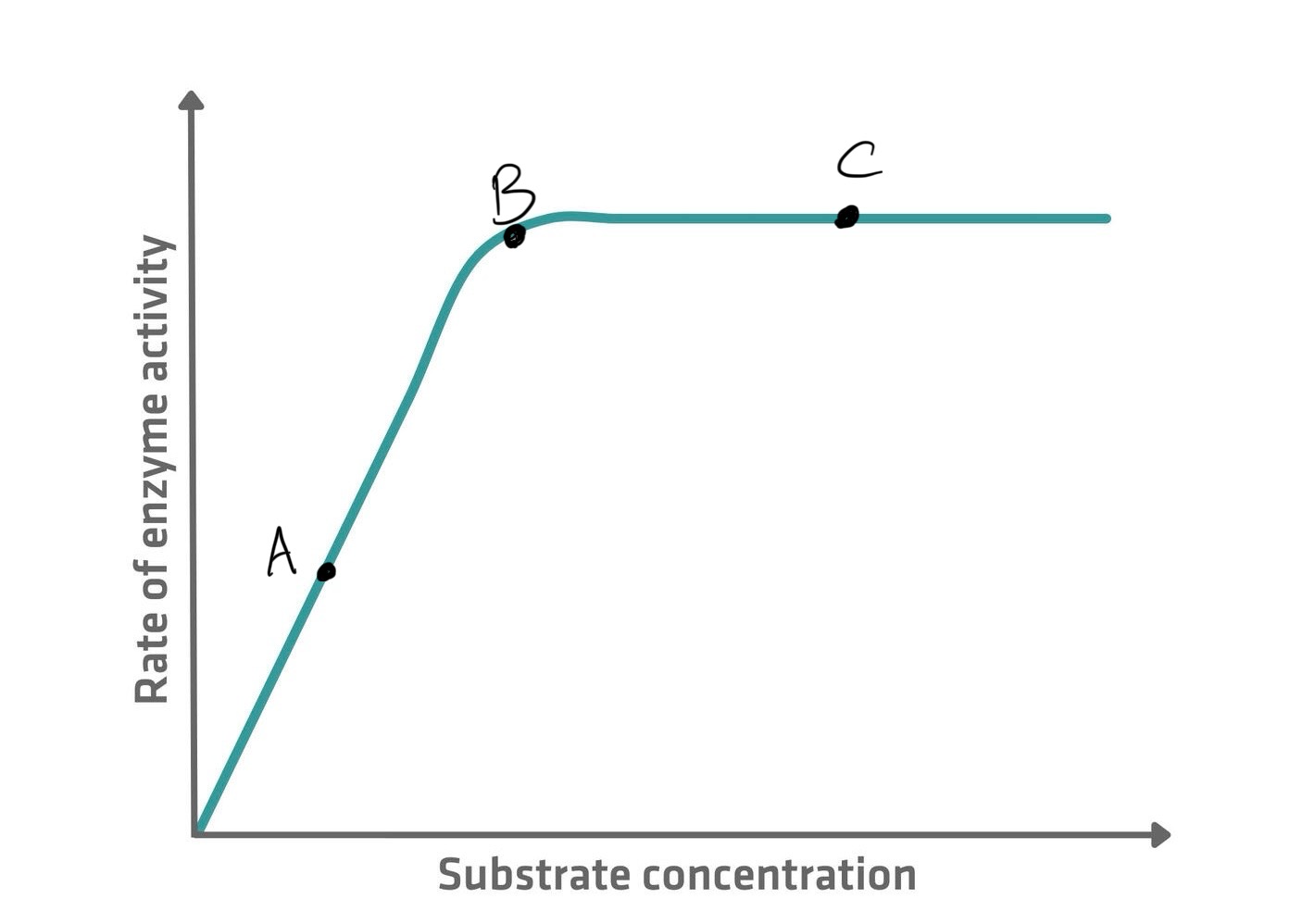
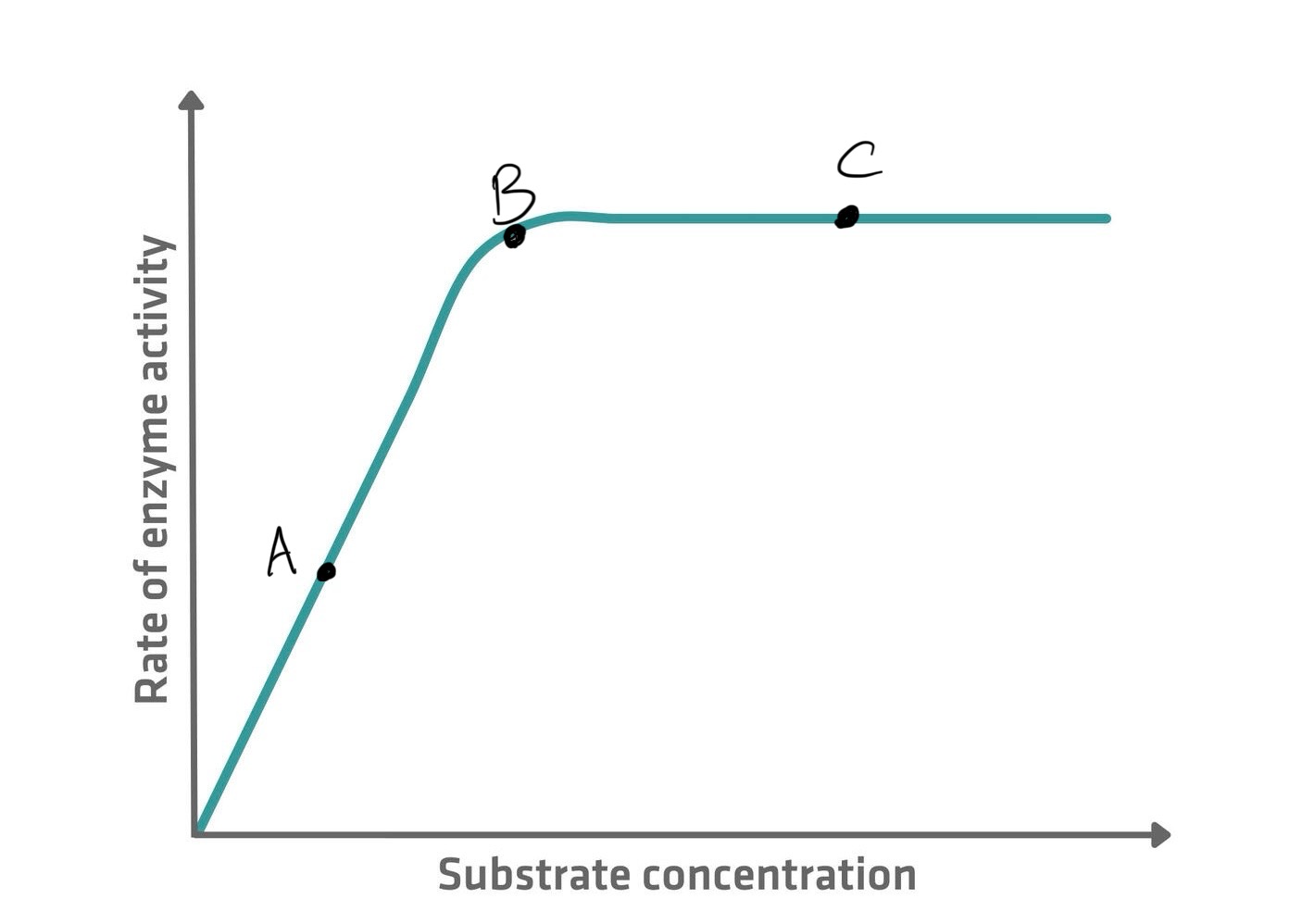
Affect of substrate concentration on enzymes
A - Too few substrate molecules to occupy all the available active sites. The rate of reaction is only half the maximum possible.
B - All the active sites are occupied at one time. The rate of reaction has doubled to its maximum because all the active sites are filled.
The addition of further substrate molecules has no effect as all active sites are already occupied at one time. There is no change in the rate of reaction.
What are enzyme inhibitors
Substances that directly or indirectly interfere with the functioning of the active site of an enzyme and so reduces its activity.
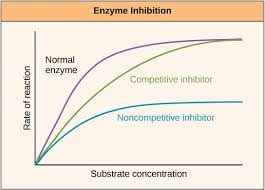
Two types of enzyme inhibitors
Competitive inhibitors - bind to the active site of the enzyme.
Non-competitive inhibitors - bind to the enzymes at a position other than the active site.
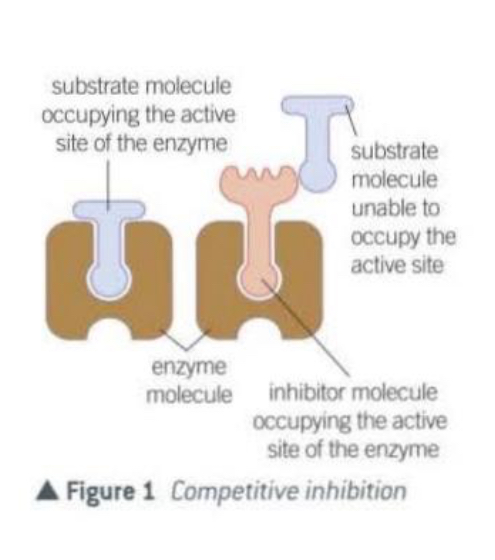
What are competitive inhibitors
Have a molecular shape similar to the substrate.
This allows them to occupy the active site of an enzyme.
They compete with the substrate for the available active sites.
The inhibitor is not permanently bound to the active site and so when it leaves, another molecule will take its place.
Sooner or later, all the substrate molecules will occupy an active site, but the greater the concentration of the inhibitor , the longer this will take.
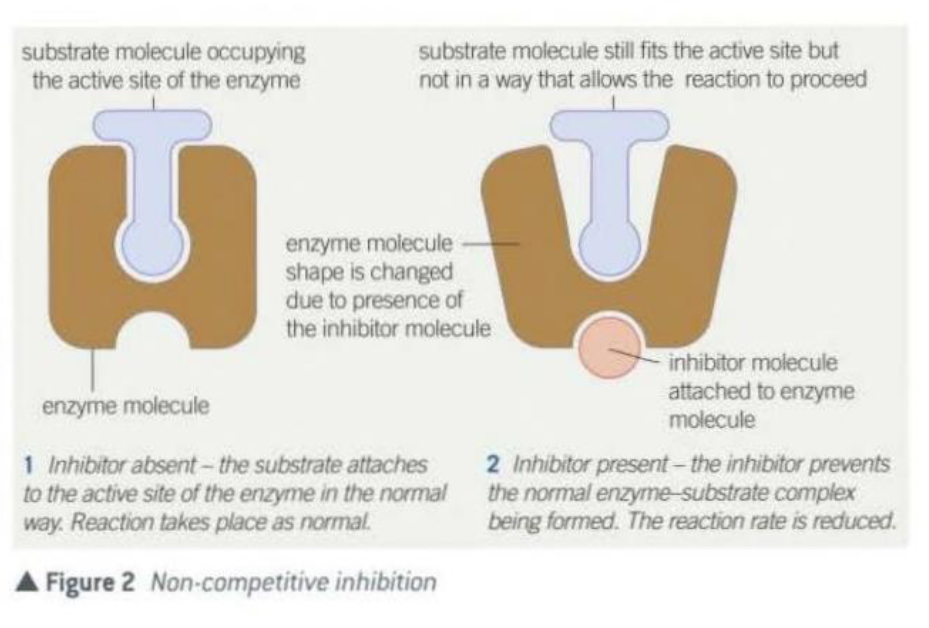
Non-competitive inhibitors
They attach themselves to the enzyme at a binding site which is not the active site.
After attaching, the inhibitor alters the shape of the enzyme and its active site in such a way that substrate molecules can no longer occupy it, they are no longer complementary to the substrate and no enzyme-substrate complex forms.
As the substrate and the inhibitor are not competing for the same site, an increase in substrate concentration does not decrease their the effect of the inhibitor.
Which molecules store and transmit genetic information in cells?
RNA - ribonucleic acid
DNA - deoxyribonucleic acid