Unit 3: IMF, Gas Laws
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
Boyle’s Law
P1V1 = P2V2
Charles’ Law
V1 V2
T1 = T2
pressure and moles are constant
Gay-Lussac’s Law
P1 P2
T1 = T2
volume and moles are constant
Avogadro’s Law
V1 V2
n1 = n2
pressure and temperature are constant
Ideal Gas Law
PV = nRT
Combined Gas Law
when number of moles of gas is constant
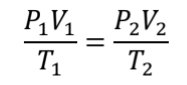
Dalton’s Law of Partial Pressures
Ptotal = PA + PB + PC …
Open system
system that allows the open transfer of energy and matter/mass
Closed system
system that allows the transfer of energy only
Isolated system
system that does not allow the transfer of either energy or matter
System
the volume that contains matter chemical and physical properties
Intensive property
Amount independent; properties with values that do not add
EX: temperature and pressure
two water samples at 100 degree C do not total 200 when poured together
Extensive property
Amount dependent; properties that do add together
EX: volume and moles
State functions
depends only on how it ends (change of final - initial)
EX: temperature, volume, pressure, moles of gas, enthalpy, entropy, internal energy, Gibbs free energy
Path function
function that keeps track of what is happening between the beginning and end
EX: heat, q, and work, w
KMT assumptions
gas particles have no volume
gas particles undergo elastic collision (KE is conserved)
gas particles have a KE that is proportional to temperature
gas particles have a velocity that varies as the inverse square of the mass
Which KMT assumptions are flawed?
Gas particles DO have volume
Gas particles can undergo collision that are inelastic (EX: water that sticks at room temp)
At room temp, which of the naturally diatomic atoms is solid at room temp?
I2
At room temp, which of the naturally diatomic atoms is liquid at room temp?
Br2
Molecular Weight
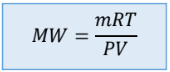
Density (also include substitution into IGL)
p = m/v
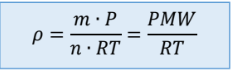
What is the volume of 1 mole of gas at STP?
22.4 L
Consider the Maxwell-Boltzmann distribution of gases, if m stays constant, then as T increases…
V increases; KE increases
Consider the Maxwell-Boltzmann distribution of gases, if T stays constant, then as m increases…
V decreases
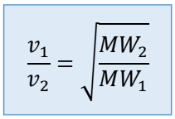
Graham’s Law of Diffusion/Effusion
What are the ideal conditions for an ideal gas?
Collisions are rare and elastic
How can you manipulate each variable of IGL to make conditions of a gas more ideal?
P is low so don't collide
V is large so don't collide
n is small so don't collide
T is large so don't stick
Consider the van der Waals correction factors, what does “a” correct?
“a” corrects for stickiness aka IMF
Large gases (molecular weight) and polar gases have large a, while small nonpolar gases have small a.
Consider the van der Waals correction factors, what does “b” correct?
“b” corrects for size
Large molecules (molecular weight) have large b, small molecules have small b.
Dispersion forces increase as
Molecular weight increases
Dipole-dipole forces increase as
The change in electronegativity increases
Hydrogen bonding increases as
the number of hydrogen bonds increase OR N-H < O-H
Ionic IMF increases as
Charge density increases (charge number and size)
Define boiling point and relationship to IMF
Bulk phenomenon in which the atmospheric pressure equals the vapor pressure of the liquid, causing the liquid to become a vapor.
Direct to IMF.
Define surface tension and relationship to IMF
Surface phenomenon in which an inward force reduces the surface area of liquid. This is why liquids like H2O bead up on windrows in the rain.
Direct to IMF.
Define capillary action and relationship to IMF
Surface phenomenon in which the liquid climbs the walls of the container because of IMF.
Direct to IMF.
Define viscosity and relationship to IMF
Tendency of a liquid to resist pouring because of IMF attraction to bulk solution.
Direct to IMF.
Define Heat of Vaporization and relationship to IMF
The energy in IMF that must be overcome for a liquid on the surface to vaporize (become gas)
direct to IMF
Define vapor pressure and relationship to IMF
Pressure of the vapor above the surface of a liquid
inverse to IMF
Define evaporation rate and relationship to IMF
How quickly a liquid will vaporize from the surface of a liquid
inverse to IMF
Order the h-bonds
NH3 < HF < H2O
NH3 - small EN even though it has 3 h bonds
HF - larger EN but only one h bond
H2O - sizeable EN and 2 h bonds