Chapter 5. Introduction to Clinical Experimentation
1/66
Earn XP
Description and Tags
research class
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
67 Terms
Evidence-Based Medicine
The practice of medicine that involves making clinical decisions based on the best available, current, valid, and relevant evidence.
Clinical Study
Any attempt to learn more about a disease and its manifestations, causes, or outcomes, varying in size from small case descriptions to large trials.
Clinical Trial
A specific type of clinical study that evaluates the effects of a specific medical or health-related intervention in humans.
Quantifiable Outcome
An outcome in a clinical trial that can be measured in a way that quantifies the effectiveness of an intervention.
Control Group
A group in a clinical trial that does not receive the treatment being tested, used as a benchmark to compare the effects of the experimental treatment.
Randomization
The process of assigning trial participants to treatment or control groups randomly to minimize bias.
Blinding
A method used in clinical trials to prevent bias by ensuring that participants and/or investigators do not know which treatment participants are receiving.
Null Hypothesis
The hypothesis that there is no effect or no difference, which is tested in a clinical trial.
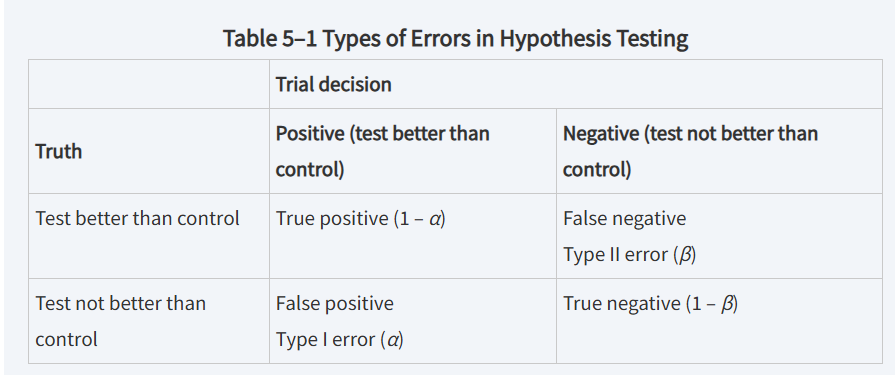
Type I Error
The error that occurs when the null hypothesis is incorrectly rejected, indicating a false positive.
Type II Error
The error that occurs when the null hypothesis is not rejected, indicating a false negative.
Phase I Trial
The first stage in clinical drug development, focusing on safety, dosage, and pharmacokinetics, often involving healthy subjects.
Phase II Trial
The stage that evaluates the effectiveness of a drug while establishing short-term side effects and risks.
Phase III Trial
A large-scale trial conducted to confirm effectiveness, monitor side effects, and compare the drug to commonly used treatments.
Phase IV Trial
Post-marketing studies done after a drug has been approved to evaluate long-term effectiveness and safety.
Statistical Significance
A statistical result that is unlikely to have occurred by chance, often indicated by a p-value less than a specified alpha level, usually 0.05.
P Value
The probability of obtaining a test statistic at least as extreme as the one observed, assuming the null hypothesis is true.
Surrogate Outcome
A measure used as a substitute for a clinically meaningful endpoint, often used in Phase II studies to infer actual clinical benefit.
scientific method
construction of a hypothesis, design of an experiment, analysis of the data, and communication of the results
retrospective studies
collect and analyze data from events that have already occurred
prospective studies
identify a population of subjects and follow them into the future from a specified time point, collecting data on events that occur as time passes
types of clinical studies
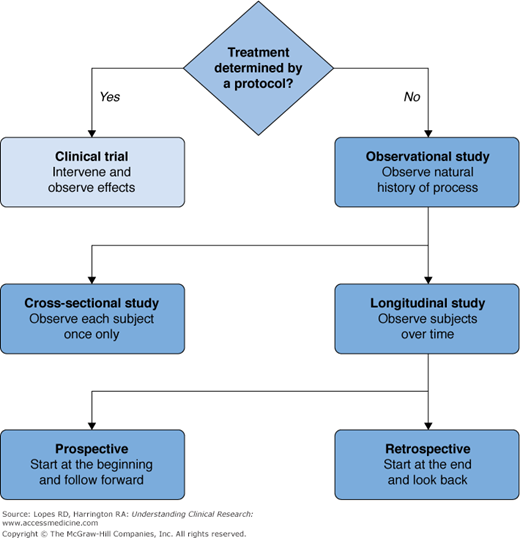
relevancy of outcome measures is also known as
content validity
content validity
a measurement validity that assesses how well a test or assessment represents a defined construct or domain of knowledge
The basis for inference from a clinical trial is
the observed difference in the summarized outcome measure(s) between a group that received an experimental treatment and a group that received the control treatment
treatment effect
a measurement that compares the outcomes of a treatment to the outcomes of not receiving the treatment
commonly used stratification factors are
sex, age, race, as well as known measures of risk observable at baseline
randomization
randomly sorting participants into groups
assures that the treatment a pt will receive is determined by chance and is unrelated to characteristics of the specific pt
blinding
when investigators and pts are unaware of which treatment is received
reduces the potential for bias in the reporting of measures taken during the study
null hypothesis
assumption that there is no difference btwn the test and control
delta=0
in a positive trial, the observed delta. . .
would be so different from 0 that we would not believe that the null hypothesis would be true
so you reject the null hypothesis
if the observed delta is not far enough from 0 . . .
then it is a negative trial
aka a failure to reject the null hypothesis
types of error
statistical significance
is reported when the computed test statistic is so large that, if the test and control arms were not really different and we were to repeat our clinical trial many times, no more that 100 x a% of the repeats would produce a test statistic as large as the one we observed
the P value is
the computed probability of observing a test statistic at least as large as the one we observed, assuming the null hypothesis of no difference between the treatment arms
statistical significance is claimed if
the P value is less than the prespecified α
clinical significance
assess the apparent net benefit of a tx for individual pts taking into account other factors such as the risk of adverse events or cost
random assignment of pts and type I error
randomization reduces the potential for bias in the treatment comparison by balancing prognostic factors between treatment groups
randomization introduces the element of chance necessary to interpret the P value as a probability statement
the value of a
the level of significance
is specified in the design of the clinical trial
a is usually set to
0.05
when a=0.05, the type I error is really
0.025 (0.05/2)
bc cases in which the control tx is significantly better than the test tx do NOT count as positive trials
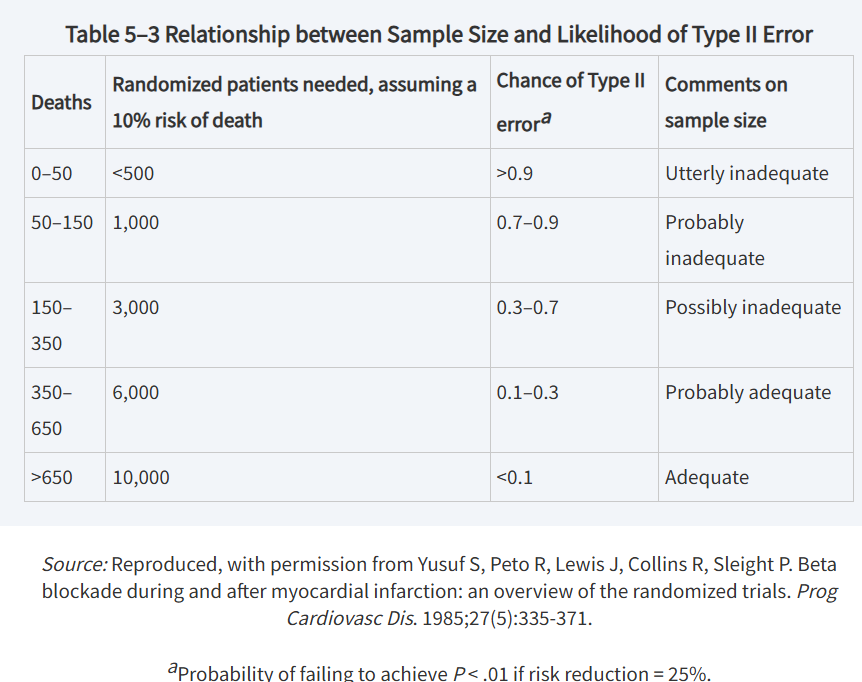
to control type II error (β)
we consider a hypothetical nonzero difference between the test and control treatment arms (ΔA = μT – μC) and the anticipated variation in ΔA (sdiff = standard deviation of ΔA)
The suggestion that ΔA > 0 is called the
alternative hypothesis
power of the trial is
1 – β and is the smallest probability of a positive trial, assuming that the true average difference between test and control treatment arms is at least ΔA
ΔA is
the smallest detectable difference between treatment arms, assuming power of at least 1 – β
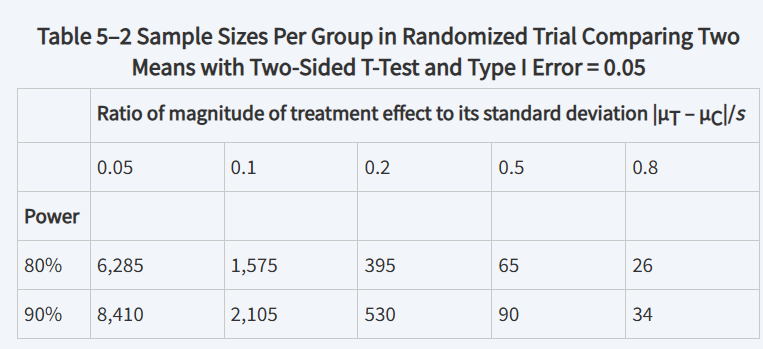
The required sample size for a trial ___________ with increasing ΔA
decreases
power is chosen to be at least _______, so that Type II error is less than ______
0.8; 0.2
Smaller Type I error, smaller Type II error, and smaller ratios require
larger sample sizes
relationship between ΔA/s and sample size
possible effects of sample size on Type II error
types of trials designed to show that tx arms are not different are
equivalence trials and noninferiority trials
equivalence trials
designed to show that two treatments give similar results
noninferiority trials
where the objective is to show that the test treatment is not worse than the control by more than a specified amount
Pharmacokinetic studies often do not have a
specified hypothesis test, as the objective of these trials is to estimate variables related to the pharmacology of the treatment
clinical development for new drugs often have _______ phases
4 phases
phase I
the drug is first introduced to humans
usually as a single dose or injection given to a small number of healthy subjects
why is knowing a drug’s half-life important?
because longer half-lives can be associated with the possibility of accumulation and increased risk of side effects
comparison of clinical trial phases
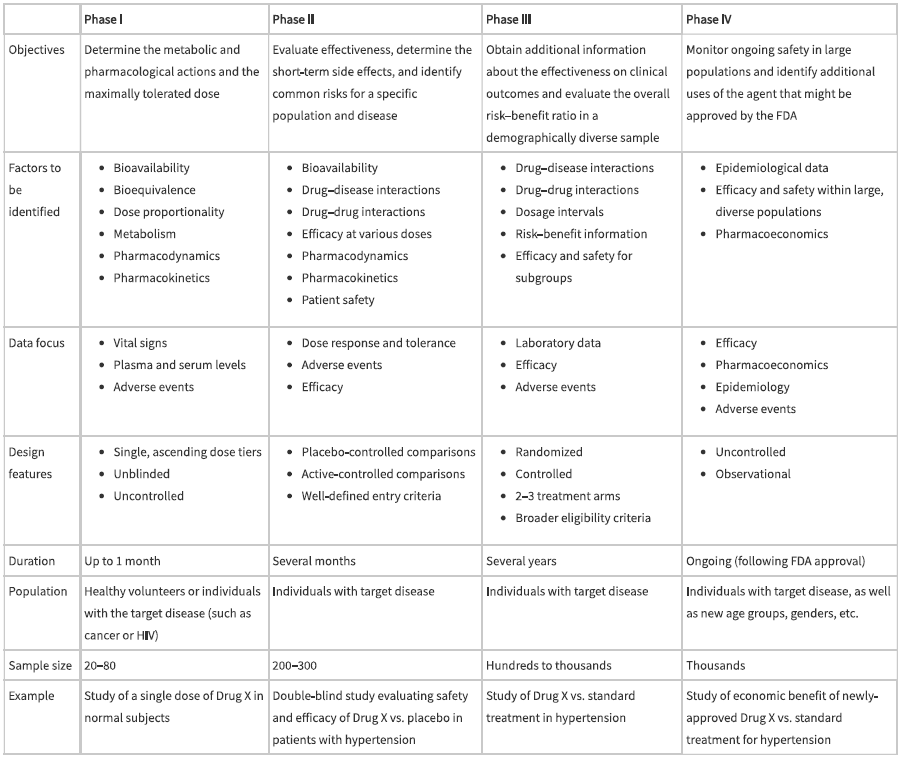
later phase I studies might explore
ADME of the drug by evaluating multiple-dose regimens, bioavailability of oral forms, and comparison of pharmacokinetics between fed and fasting conditions
phase II studies
begin to establish evidence of drug effect for its intended indication
phase IIa should identify a
“target” dose at which maximal efficacy is expected to be observed
phase IIb trials
are randomized and have sample sizes large enough to obtain more precise estimates of efficacy and adverse event rates
where the target dose is tested
An important goal of drug development is to show
that the test treatment is statistically significantly better than the control with respect to outcome measures reflecting clinical benefit, such as survival, time to adverse outcome, disease symptom score, or functional status
surrogate outcomes
a physical sign or laboratory measurement that can be used to predict a clinically important outcome
Surrogate outcomes are used in clinical trials when it's not possible or practical to measure the clinical outcome directly
phase III
where demonstration of clinical benefit is accomplished
phase IV testing obtains
further evidence regarding safety, such as effects of chronic treatment or adverse event profiles in subgroups