The equilibrium constant Kc
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
How do you work out Kc?

What must you bear in mind when calculating Kc?
You must only use the same state
What does it mean if the Kc value is 1?
A position of equilibrium that is halfway between reactants and products
What does it mean if the Kc is greater than 1?
A position of equilibrium that is towards the products
What does it mean if the Kc value is less than 1?
A position of equilibrium that is towards the reactants
What is homogeneous equilibrium?
A homogenous equilibrium contains equilibrium species that all have the same phase
What is heterogeneous equilibrium?
A heterogenous equilibrium contains equilibrium species that have different phases
What do the square brackets represent?
Mol dm-3
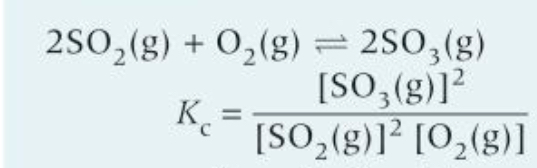
How would you work out the units of this?
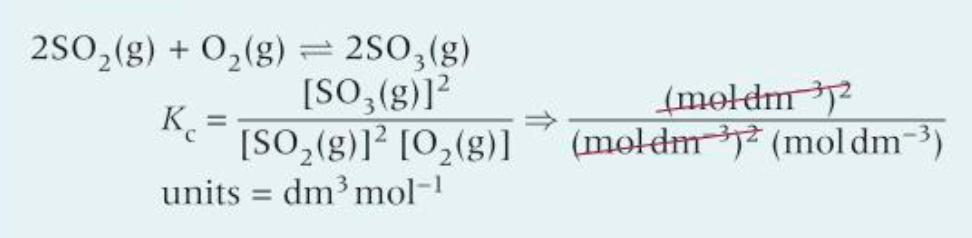
What effects Kc?
ONLY temperature