CAIE Chemistry IGCSE: Formulae and Calculations
1/64
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
65 Terms
Liquid Substances at Room Temperature
H2O (l) - Pure water, Br2 (l) - Bromine, Hg (l) - Mercury.
Solid Substances at Room Temperature
Most metals are solid at room temperature, e.g., Na (s), Mg (s), Fe (s), and iodine I2.
Empirical Formula
The smallest whole number ratio of the atoms of each element in a compound.
Relative atomic mass (Ar)
The relative atomic mass, Ar, of an element is the average mass of the isotopes of an element compared to 1/12th of the mass of an atom of carbon-12.
Relative formula mass (Mr)
The sum of the relative atomic masses of atoms in a formula unit. Used for giant ionic structures.
Calculate mass of magnesium for magnesium oxide
1. Find the Mr of magnesium: 24; 2. Find the Mr of magnesium oxide: (Ar of Mg is 24 and Ar of oxygen is 16); 3. Find the mol of magnesium oxide: mass of MgO ÷ Mr of MgO; 12 ÷ 40= 0.3; 4. The moles of magnesium is also 0.3 since the balancing numbers of Mg and MgO are the same.
Mass of magnesium
Mr of Mg x mol of Mg
Concentration
The concentration of a substance is the amount of solute dissolved in a measured volume of solution. The concentration can be measured in g/dm3 or mol/dm3.
Mole
The mole is the unit for amount of any substance, containing the same number of particles as there are atoms in exactly 12 g of carbon-12 (1 mole= 6.02 x 10^23 particles).
Avogadro constant
The number of particles in one mole of a substance. This is 6.02 x 10^23 particles.
Equation relating moles and Avogadro constant
Number of particles = moles x Avogadro's constant.
Equation relating amount of substance, mass and molar mass
Mass of a substance (in g) = Moles (mol) x Molar mass of substance(g/mol).
Molar mass
The molar mass is the mass of 1 mole of the substance. It is the same as the Ar / Mr of the substance but the molar mass has a unit: g/mol.
Amount of substance in 300g of calcium carbonate (CaCO3)
1. Calculate the Mr of CaCO3: 40 + 12 + (16 x 3) = 100. 2. Mol= Mass ÷ Mr: 300 g ÷ 100 = 3 moles of CaCO3.
Mass of 0.75mol of calcium (Ca)
1. Find the Mr of Ca on periodic table = 40. 2. Mass= Mr x mol: 40 x 0.75 = 30g.
Moles in 5.44 g of sodium chloride
Mr of NaCl = 23 + 35.5 = 58.5. Moles = mass / Mr: = 5.44 / 58.5 = 0.0930 (3.s.f).
Moles in 2.35 g of aluminium
To be calculated based on the molar mass of aluminium.
Moles
mass / relative atomic mass
Molar mass of AlCl3
534 g ÷ 4 mol = 133.5 g/mol
Mr of AlCl3
27 + (3 x 35.5) = 133.5
Number of particles
Avogadro constant x amount of substance
Number of CO2 molecules
6.02 x 10^23 x 1.5 = 9.03 x 10^23
Number of atoms in 1.5 moles of CO2
9.03 x 10^23 x 3 = 2.71 x 10^24 atoms
Molar volume of gas at room temperature and pressure
24 dm3
RTP
Room temperature and pressure: 20oC, 1 atmosphere
Equation linking molar volume at RTP and moles
Volume of gas at RTP (dm3) = moles x 24dm3
Moles of oxygen in 72 dm3 at RTP
72 / 24 = 3 moles
Limiting reagent
The reactant that is completely used up first, preventing the reaction continuing and determines the amount of product that can form.
Mass of magnesium chloride formed
Moles of Mg = 0.953 / 24 = 0.0397; Mass of MgCl2 = 0.0397 x (24 + 35.5 + 35.5) = 3.77 g (3.s.f)
Convert between cm3 and dm3
1dm3 = 1000cm3; Converting cm3 to dm3 : ÷1000; Converting dm3 to cm3 : x1000
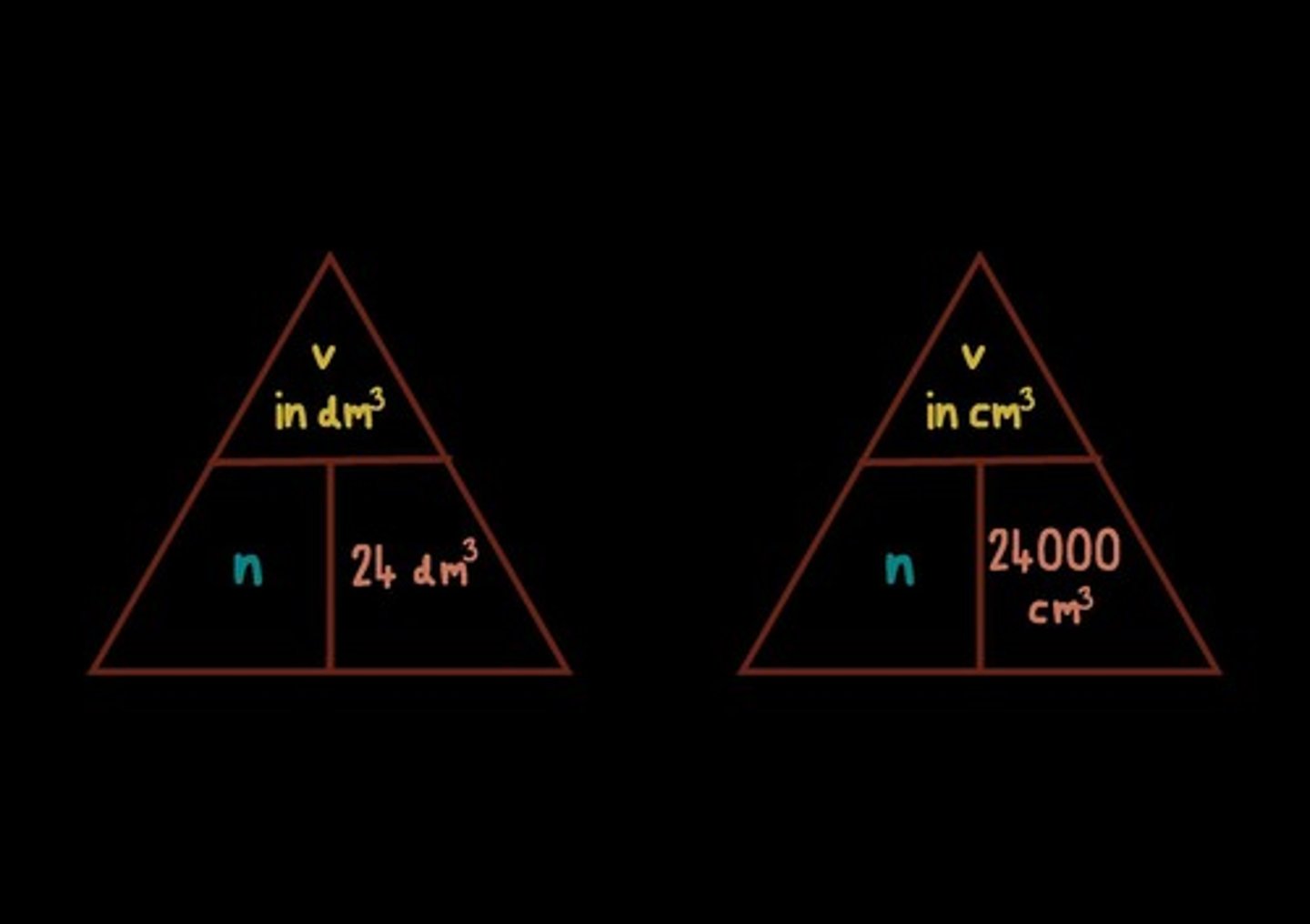
Volume of 0.5mol of hydrogen at room temperature and pressure
Volume of gas in cm3 = Number of moles x 24000cm3; Volume of hydrogen= 0.5 x 24000 = 12000 cm3
Equation linking concentration, volume and amount of substance
Concentration (in mol/dm3) : Amount of substance (mol) / Volume (dm3)
Convert between mol/dm3 and g/dm3
mol/dm3 -> g/dm3 multiply by the Mr; g/dm3-> mol/dm3 divide by the Mr
5.00 g of NaCl
is dissolved in 25 cm3 of water.
mol/dm3 to g/dm3
Multiply by the Mr.
g/dm3 to mol/dm3
Divide by the Mr.
Concentration of NaCl solution
3.42 mol/dm3.
Moles of NaCl calculation
5 / 58.5 = 0.0855.
Volume in dm3 calculation
25 cm3 = 0.025 dm3.
Concentration of hydrochloric acid
0.4 mol/dm3.
Moles of NaOH calculation
0.5 x 0.02 = 0.01 mol.
Mole ratio of HCl to NaOH
1:1 ratio.
Molecular formula from empirical formula
C8H6O4.
Empirical Mr calculation
(12 x 4) + (1 x 3) + (16 x 2) = 83.
Relative molecular mass comparison
166 / 83 = 2.
Empirical formula from percentage masses
AlCl3.
Relative atomic mass of Al
27
Relative atomic mass of Cl
35.5.
Moles of Al calculation
20.2 ÷ 27 = 0.748.
Moles of Cl calculation
79.8 ÷ 35.5 = 2.248.
Molar ratio of Al to Cl
1:3.
Empirical formula from mass of Iron and Oxygen
Fe2O3.
Moles of Iron calculation
7.83g ÷ 56 = 0.14.
Moles of Oxygen calculation
3.37 ÷ 16 = 0.21.
Theoretical yield definition
The maximum amount of product that would be collected under perfect reaction conditions.
Reasons for not obtaining theoretical yield
1. Reaction may not go to completion because it is reversible. 2. Some of the product may be lost when it is separated from the reaction mixture.
Percentage yield
Percentage yield = Actual yield x 100 / Theoretical yield
Percentage yield of NH3
Percentage yield = (40.5/227) x 100 = 17.9%.
Moles of ammonia
Moles of ammonia = 20/1.5 = 13.3 moles.
Mass of ammonia
Mass of ammonia = 13.3 x (14+1+1+1) = 227 g.
Percentage composition of an element
Percentage mass = Total Ar of the element x 100 / Mr of the compound.
Percentage of calcium in calcium hydroxide
% mass: (40 ÷ 74) x 100 = 54.1 %.
Molar mass of calcium hydroxide
Mr of Ca(OH)2: 40 + (16 x 2) + (1 x 2) = 74.
Percentage purity
Percentage purity = Mass of the pure substance x 100 / Mass of the sample.
Percentage purity of NaCl
% purity of NaCl = 0.64g x 100 / 100g = 0.64%.