Physics 2 - Lesson 9: Rate Laws Part I
1/22
Earn XP
Description and Tags
Physics 2 Lesson 9
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
Lesson 9: Rate Laws Part I
Lesson 9: Rate Laws Part I
What equation can be used to give us the rate of a reaction (in general)?
HINT: Rate = change in _______ / change in ________.
Rate = change in concentration / change in time
Struggling to keep your MCAT equations straight? Simply conquer the 100 most important equations using Andrew's 100 Most Essential Equations Mastery Course @ https://mcatselfprep.com/course/andrews-equation-mastery-course/
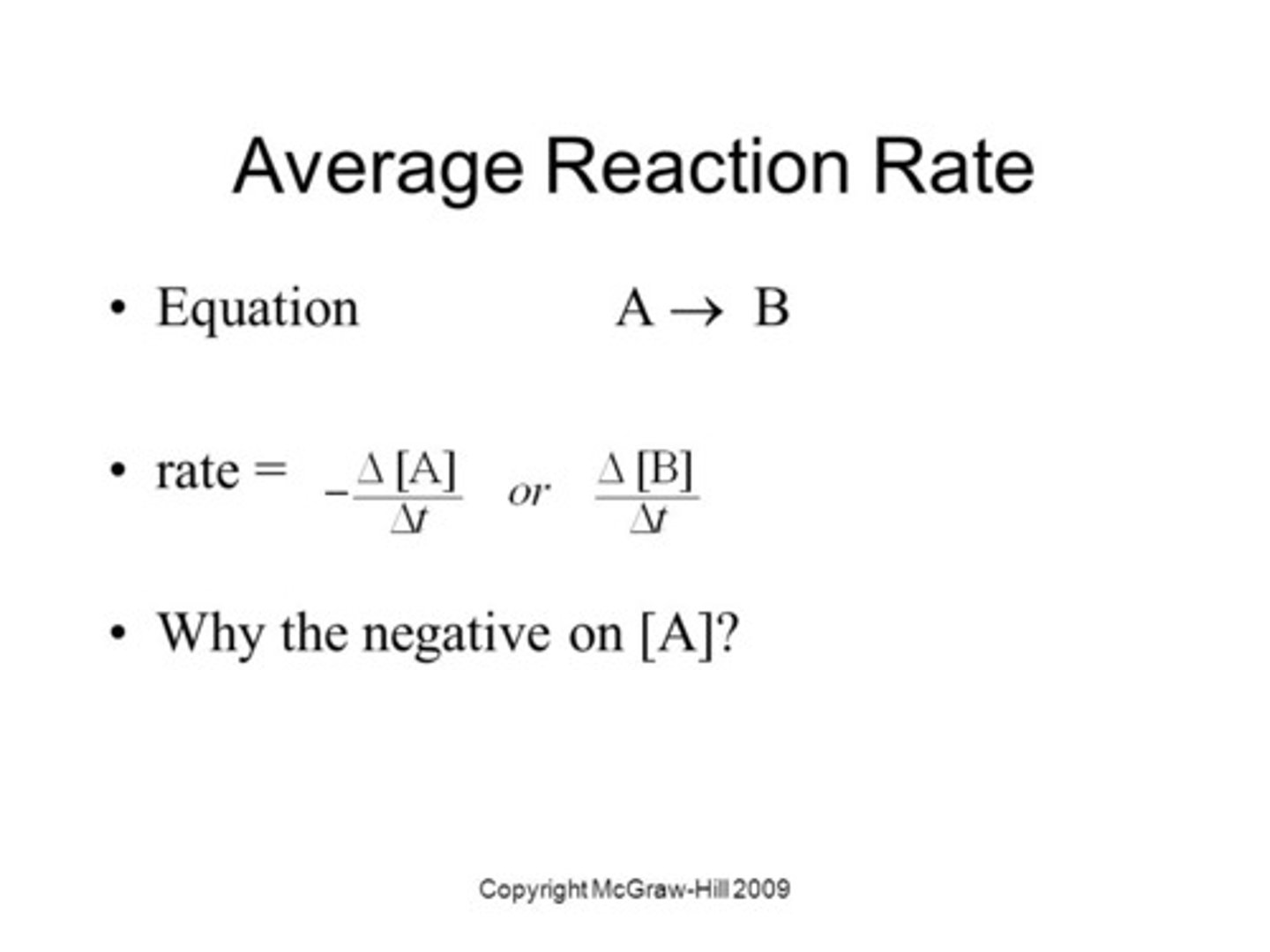
CRB In reactions with multiple elementary steps and intermediates before reaching the final product, which of the following statements about Rate Laws are true?
I. There will be a single Rate-Determining Step for the entire chain of reactions, which determines the overall rate of reaction.
II. The step with the most moving atoms will be the rate determining step.
III. The Rate-Determining Step is inconsequential when the rest of the steps happen almost instantaneously.
(A) I only
(B) III only
(C) I and II only
(D) II and III only
(A) I only
There is a single Rate-Determining Step for the entire chain of reactions, which determines the overall rate of reaction.
CRB True or false? The Rate-Determining Step is able to control the rate of the overall reaction by acting as a kinetic bottleneck, preventing the speed of the reaction being faster than the slowest step involved.
True. The Rate-Determining Step is able to control the rate of the overall reaction by acting as a kinetic bottleneck, preventing the speed of the reaction being faster than the slowest step involved.
CRB Recall Glycolysis from the Biochemistry II Module. Which of the following was the Rate-Determining Step there?
(A) Glucose + ATP --> Glucose-6-P
(B) Fructose-6-P + ATP --> Fructose-1,6-Bisphosphate
(C) Glucose-6-P --> Fructose-6-P
(D) Phosphoenolpyruvate (PEP) + ADP --> Pyruvate + ATP
(B) Fructose-6-P + ATP --> Fructose-1,6-Bisphosphate
Need help keeping track of different biochemical pathways? Check out Andrew's Metabolic Pathways Mastery Course! https://mcatselfprep.com/course/andrews-metabolic-pathways-mastery-course/
CRB Of the following compounds in the Glycolysis pathway, which of them would be classified as intermediates?
- Glucose
- Pyruvate
- Fructose 1,6-Bisphosphate
- Phosphoenolpyruvate (PEP)
- Fructose-6-P
-Glucose-6-P
-ATP
- Fructose 1,6-Bisphosphate
- Phosphoenolpyruvate (PEP)
- Fructose-6-P
-Glucose-6-P
Note that intermediates are formed in one reaction and subsequently used in the following reaction in the chain, so that intermediates would cancel out in a net equation.
CRB Which of the following would NOT determine the rate of a reaction?
(A) The energy of the reagents
(B) The orientation of the colliding molecules
(C) The size of the reagents
(D) How frequently the reagent molecules collide
(C) The size of the reagents
The 3 main factors determining a reaction rate are:
I. The energy of the reagents
II. The orientation of the colliding molecules
III. How frequently the reagent molecules collide
Consider the following equation: A -> B. If A's concentration goes from 4.5 M to 2.4 M in 6.7 seconds, what is the rate for the formation of B (in M/s)?
(A) .0313
(B) .313
(C) 3.13
(D) 31.3
(B) .313
Rate = change in concentration / change in time
Rate = (4.5 - 2.4) / 6.7
Rate = 2.1 / 6.7
Rate = approx. .33 (actual: .313)
Need help with MCAT math? Become an MCAT math wizard using Andrew's High-speed Math Mastery Course @ https://mcatselfprep.com/course/andrews-high-speed-math-mastery-course/
True or False. Reaction Order can be determined by simply looking at the chemical reaction formula.
False. Reaction Order must be determined experimentally.

CRB Which of the following statements about Activation Energy are true?
I. Activation energy is correlated to the Gibbs Free Energy difference between reagents and products.
II. Activation energy is the minimum energy needed for the reagent molecules in a collision to proceed in the reaction to form products.
III. The Transition State, or Activated Complex, has the highest free energy during this reaction process, and the Activation Energy is the difference between this Free Energy of the Transition State and the Free Energy of the Reagents.
(A) I only
(B) III only
(C) II and III only
(D) I, II and III
(C) II and III only
Each of the following statements about Activation Energy are true:
II. Activation energy is the minimum energy needed for the reagent molecules in a collision to proceed in the reaction to form products.
III. The Transition State, or Activated Complex, has the highest free energy during this reaction process, and the Activation Energy is the difference between this Free Energy of the Transition State and the Free Energy of the Reagents.
CRB Which of the following would introducing an enzyme affect?
(A) Gibbs Free Energy difference between Reagents and Products
(B) Transition State Stability
(C) Time spent as Intermediate
(D) None of the above
(B) Transition State Stability
Increasing the Transition State Stability is the chief role of enzymes in a reaction.
CRB True or false? Increasing the Transition State Stability both decreases the Gibbs Free Energy of the Transition State and the Activation Energy of the reaction.
True. Increasing the Transition State Stability both decreases the Gibbs Free Energy of the Transition State and the Activation Energy of the reaction.
What is the overall reaction order for this reaction, considering the following data: https://i.ytimg.com/vi/tSI8vcM-9Kw/hqdefault.jpg
(A) 1
(2) 2
(3) 3
(4) 4
(3) 3
When B is doubled, the reaction rate quadrupling; thus, B must have a order of 2.
When A is doubled, the rate doubles, meaning that A must have a order of 1.
This makes the overall rate law = k[A]^1[B]^2; thus the overall reaction order is 3.
Need help with MCAT math? Become an MCAT math wizard using Andrew's High-speed Math Mastery Course @ https://mcatselfprep.com/course/andrews-high-speed-math-mastery-course/
What is the value of k (using the data from experiment 1) for the following reaction: https://image.slidesharecdn.com/initialratemethod-131202005326-phpapp02/95/initial-rate-method-1-638.jpg?cb=1385945648
(A) 3.21⋅10^-3
(B) 1.73⋅10^-3
(C) 1.73⋅10^3
(D) 3.21⋅10^3
(C) 1.73⋅10^3
When [NO] is doubled, the reaction rate more than doubles, almost quadrupling; thus, [NO] must have a order of 2.
When [O2] is doubled, the rate doubles, meaning that [O2] must have a order of 1.
This makes the overall rate law = k[O2]^1[NO]^2; thus the overall reaction order is 3.
Now using the data from experiment 1:
rate = k[O2]^1[NO]^2
3.21⋅10^-3 = k(1.1⋅10^-2)(1.3⋅10^-2)^2
3.21⋅10^-3 = k(1.1⋅10^-2)(approx. 1.6⋅10^-4 (actual: 1.69⋅10^-4))
(3.21⋅10^-3) / (approx. 1.8⋅10^-6 (actual: 1.859⋅10^-6) = k
k = approx. 2⋅10^3 (actual: 1.73⋅10^3)
Need help with MCAT math? Become an MCAT math wizard using Andrew's High-speed Math Mastery Course @ https://mcatselfprep.com/course/andrews-high-speed-math-mastery-course/
What is the integrated rate law for a zero-order, first-order, and second-order reaction?
Struggling to keep your MCAT equations straight? Simply conquer the 100 most important equations using Andrew's 100 Most Essential Equations Mastery Course @ https://mcatselfprep.com/course/andrews-equation-mastery-course/
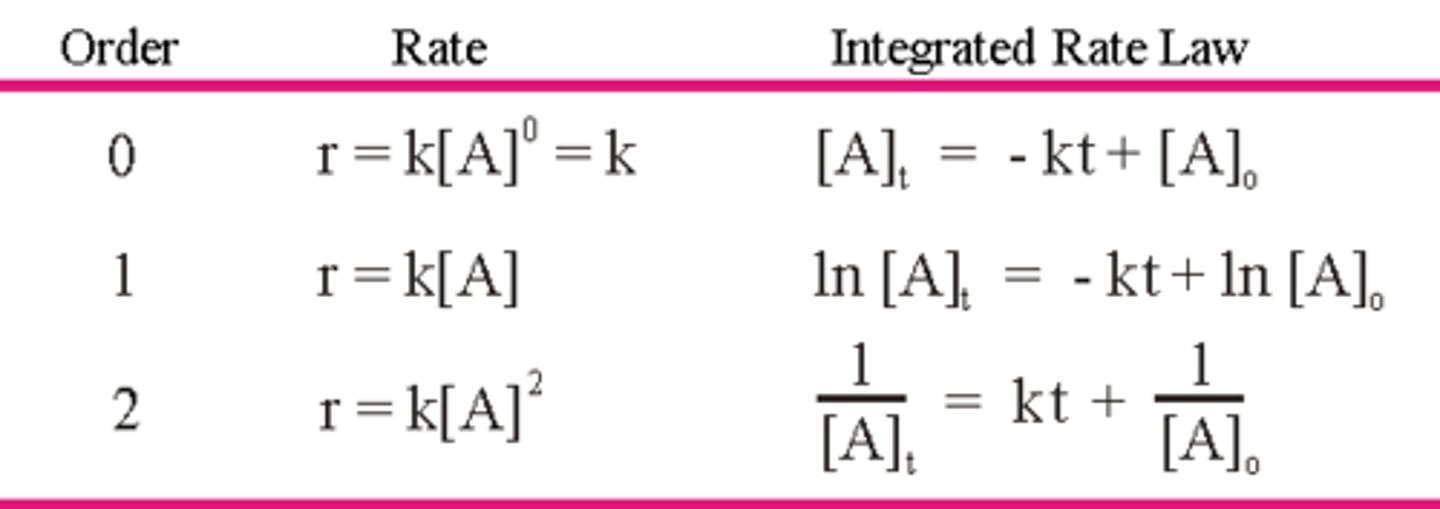
Draw the graph over time for a zero-order, first-order, and second-order reaction. Be sure to label the y-axis and state what the slope is equal to for each.
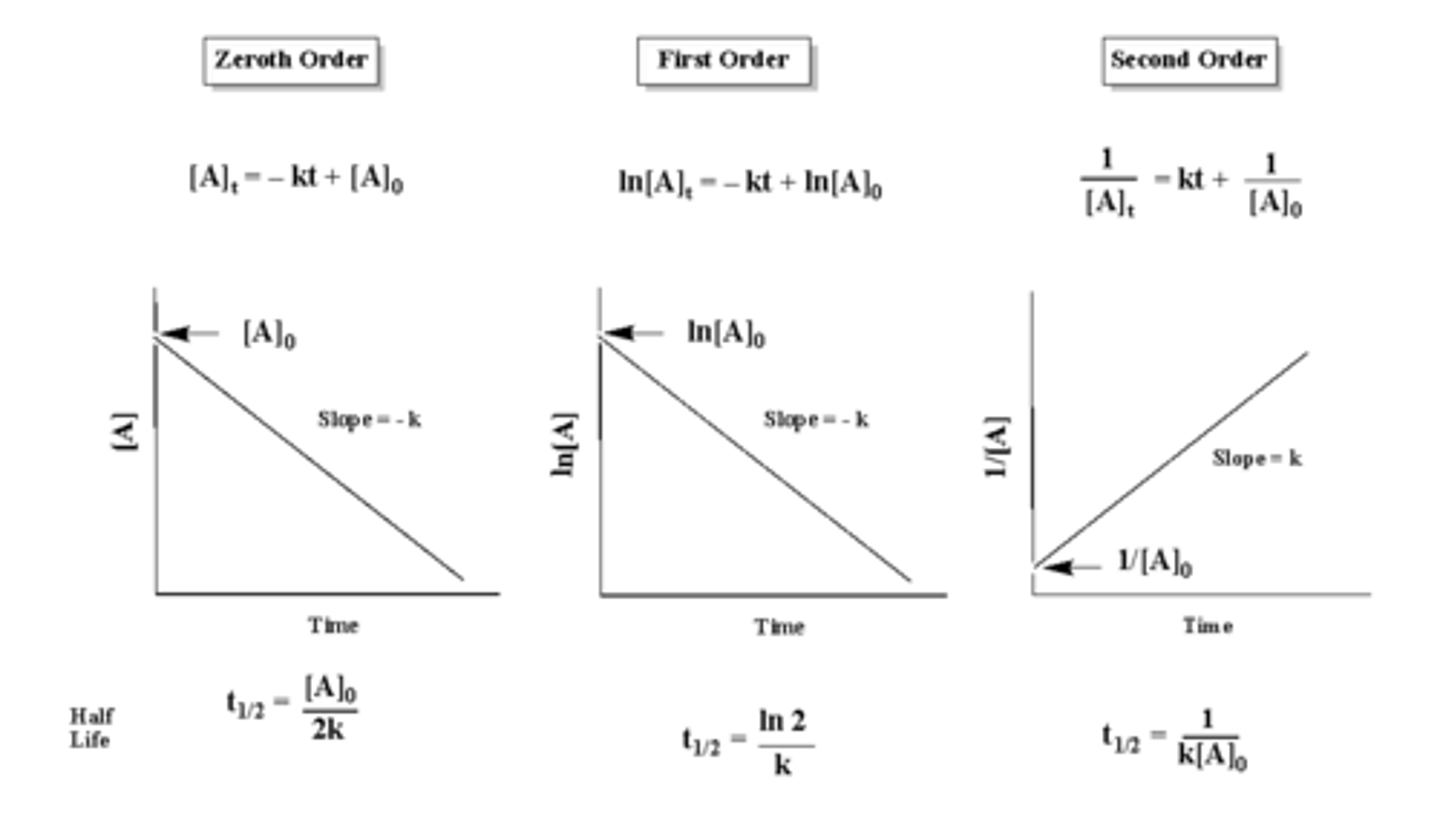
CRB True or false? There are also Mixed-order and Broken Order Reactions, where the rate of product formation looks like a Hyperbola.
False. There are also Mixed-order and Broken Order Reactions, where the Orders are Non-Integers (usually fractions for Broken Order Reactions).
Now, draw a concentration vs. time graph for a zero-order, first-order, and second-order reaction.
CRB Which of the following would NOT increase the reaction rate (not accounting for enzymes)?
(A) Increase Reagent Concentration
(B) Lowered Activation Energy
(C) Higher Temperatures of Reaction Conditions
(D) Increased Product Concentration.
(D) Increased Product Concentration.
Each of the following would increase Reaction Rates:
I. Increase Reagent Concentration
II. Lowered Activation Energy
III. Higher Temperatures of Reaction Conditions
CRB True or false? The rate of a reaction tends to double for every 100 degrees Celsius increase.
False. The rate of a reaction tends to double for every 10 degrees Celsius increase. That's really fast!
CRB In a biological system, reaction rates will tend to decrease sharply once the temperature increases over 40 degrees Celsius. Explain the reasoning for this.
Above 40 degrees Celsius, the enzymes for most biological systems will denature, and the lack of enzyme function greatly decreases the overall reaction rate in the system.
CRB Fill in the blanks: Generally, there are two types of catalysts. _________ Catalysts are in the same phase (gas/liquid/solid) as the reagents, whereas ________ Catalysts are in a different phase.
(A) Enzymatic, Artificial
(B) Homogeneous, Enzymatic
(C) Homogeneous, Heterogeneous
(D) Enzymatic, Heterogenous
(C) Homogeneous, Heterogeneous
Generally, there are two types of catalysts. Homogeneous Catalysts are in the same phase (gas/liquid/solid) as the reagents, whereas Heterogeneous Catalysts are in a different phase.
Consider the following reaction: N2 + 2O2 -> 2NO2 The half-life for this first order reaction is equal to 1.43 second. If you started with 14.3 moles of N2, and you now have 2.4 moles, how much time has passed (in seconds)?
(A) .34
(B) 1.78
(C) 3.65
(D) 4.88
(C) 3.65
(t=0) 14.3
(t=1.43) 7.15
(t = 2.86) 3.575
2.4 moles occurs sometime in here
(t = 4.29) 1.7875
For this reason, t = between 2.86 and 4.29.
NOTE: You can use the equation, but it will take you 10x longer. Estimation is always the better option for half-life questions on the MCAT!
Need help with MCAT math? Become an MCAT math wizard using Andrew's High-speed Math Mastery Course @ https://mcatselfprep.com/course/andrews-high-speed-math-mastery-course/