cell cultures 2
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
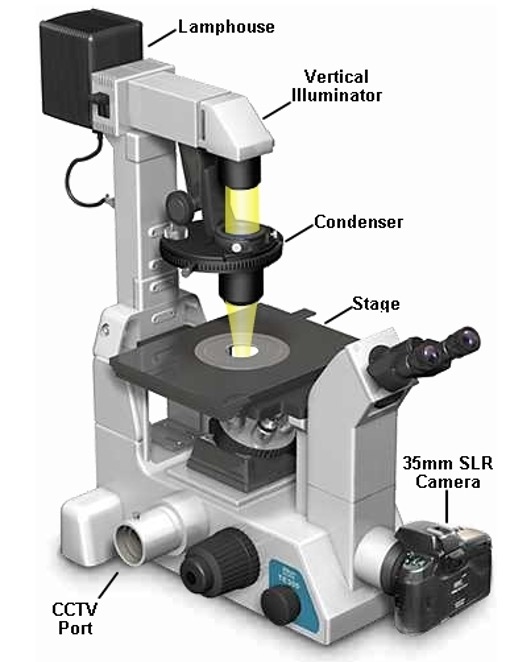
microscope used for counting cells
standard light microscope

microscope used for inspecting cell cultures
inverted microscope
what do we do with the cells
Cell sub-culturing (cell splitting) – transferring a part of the culture to a new flask and adding fresh medium
Cell plating – transferring diluted cell suspension onto a 6/12/24/96 well plate
- for experiments, not for routine culture
Cell treatment – adding a substance to cell culture medium to achieve given concentration
cell splitting/passaging
Transferring a portion of cells into a new flask
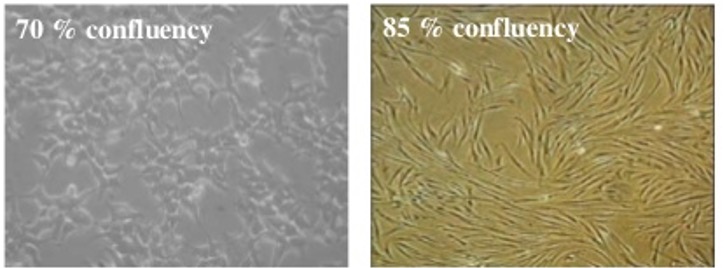
confluence
refers to adherent cells only;
•an estimate of cell number in a culture flask, by looking at the proportion of the surface area covered by cells.
•Cells show different growth rate and/or gene expression depending on the degree of confluence.
•In a series of experiments cells should be plated at the same density for consistency and reproducibility.
•When cells are approx. 70% confluent – time to split.
sub culturing cells method
1. Assess culture – medium colour & clarity, cell density and “general look”
2. Remove spent medium
3. Wash cells with PBS
4. Add trypsin*, incubate for 2-3 minutes at 37ºC
5. Check cells for detachment
6. Resuspend in fresh medium to achieve single cell suspension
(if plating cells for experiment perform cell counting)
7. Transfer desired volume to a new flask/dish/plate, add fresh medium if necessary. Label the flask (initials, date, cell line).
counting cells method
ØNeed single cell suspension (step 6 from previous slide)
ØTransfer 100 ml into Eppendorf tube
ØMix with 100 ml trypan blue
ØTransfer onto haemocytometer
ØCount live and dead cells separately to establish % viability
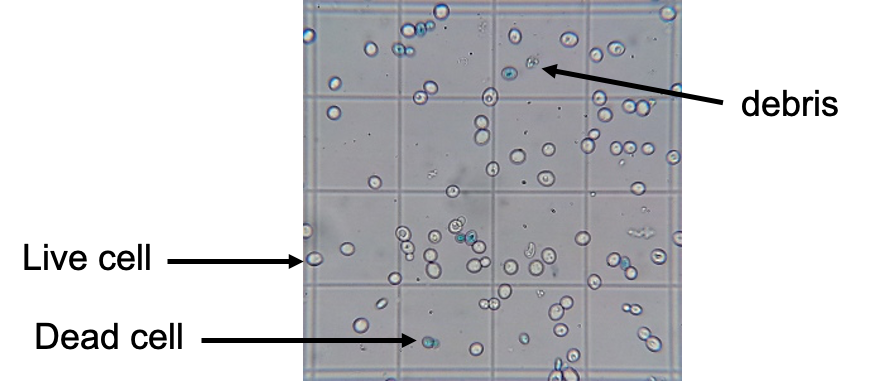
how trypan blue is used to count cells
§Trypan Blue – dye that gets inside cells*;
§Live cells expel it via membrane pumps - no colour;
Dead cells have non-functional membranes, so the dye stays in – blue cells
safety cabinet
Laminar flow of filtered air provides nearly sterile atmosphere inside
Protection for the cells, but also for the researcher
Aseptic technique still of vital importance
cell culture medium conditions
Warm
At physiological pH
Well oxygenated
Contains a significant amount of easily utilised metabolic substrates
= ideal environment for micro-organisms!
effects of contamination
Adverse effect on the culture
Inaccurate or erroneous experiment results – validity of work!
Loss of valuable products
Loss of time, money and effort
Compete for nutrients with our cells;
Secrete acidic or alkaline by-products;
Produce H2O2 which is directly toxic to cells;
Degrade arginine and purine residues;
Can change phenotype of the cells we culture
symptoms of contamination
-Change in cell growth rates
-Change in cell morphology
- Turbid culture media
- Change in pH: high or low pH (medium colour)
- Cell lysis
types of contamination
1) bacterial
2) fungal
4) yeast
3) mycoplasma
5) viral
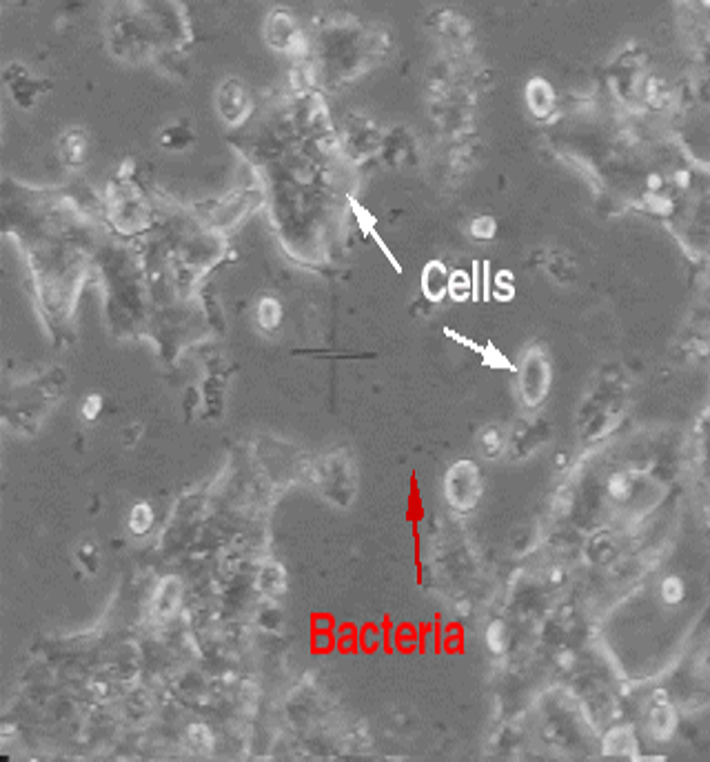
bacterial
Usually indicated by a pH change in the medium
Acidic pH = pink medium ® lemon yellow
Often turbid medium
Large scale contamination can be seen under the microscope (e.g. motile particles between the cells)
bacterial contamination remedy
autoclave the flask and discard,
unless it is a rare and difficult to obtain culture (salvage strategies)
fungal
pH usually acidic (but may be alkaline)
May be seen as fungal hyphae
Significant contamination can be seen with the naked eye
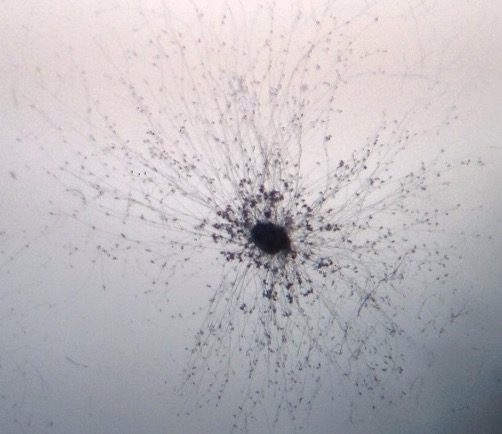
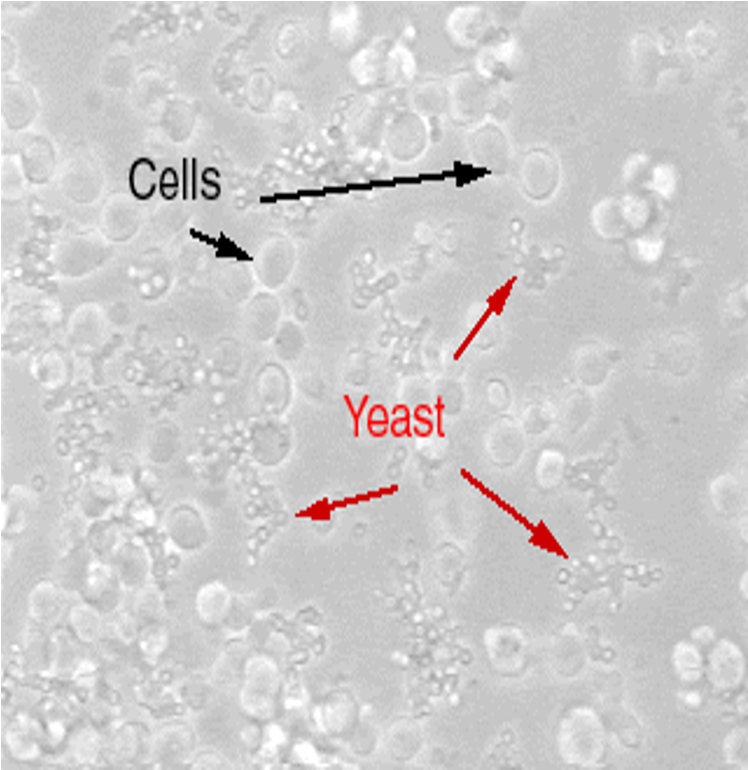
yeast
Appear as round or ovoid particles;
May be seen to ‘bud’ from other particles or form short chains
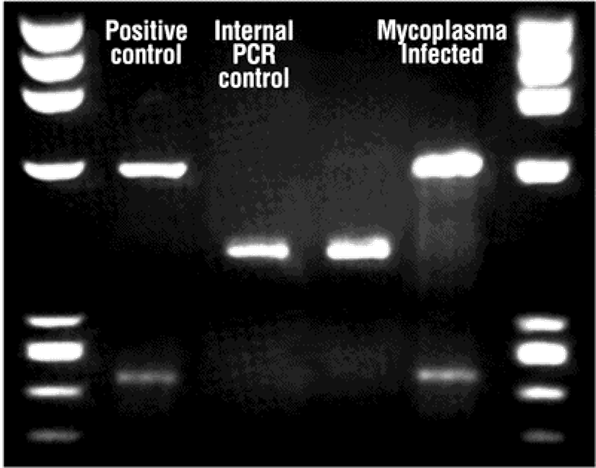
mycoplasma
The simplest and smallest bacteria
lacking a rigid cell wall = resistant to common antibiotics
size 0.1 – 0.25 µm
intracellular parasites of animal cells
may alter the characteristics of the culture: slow growth, changed response to stimuli, increased rate of mutations, etc.
•Too small to see using light microscope
•No visible change in medium
•Relatively common problem.
mycoplasma detection
•Testing should be done regularly (e.g.fortnightly)
•A few methods: PCR, colorimetric and fluorescence
•PCR using primers specific for mycoplasma
- small sample of the culture medium as a starting material
viral
Is sometimes a problem where primary cells are being used;
Some viruses are cytopathogenic – damaging to the cells;
Viruses may be suspected following abnormal culture behavior, morphological changes or cell lysis;
Difficult to detect.
if contamination is detected the affected flask must be disposed of immediately.
It is important to try to identify the source to minimise future occurrences.
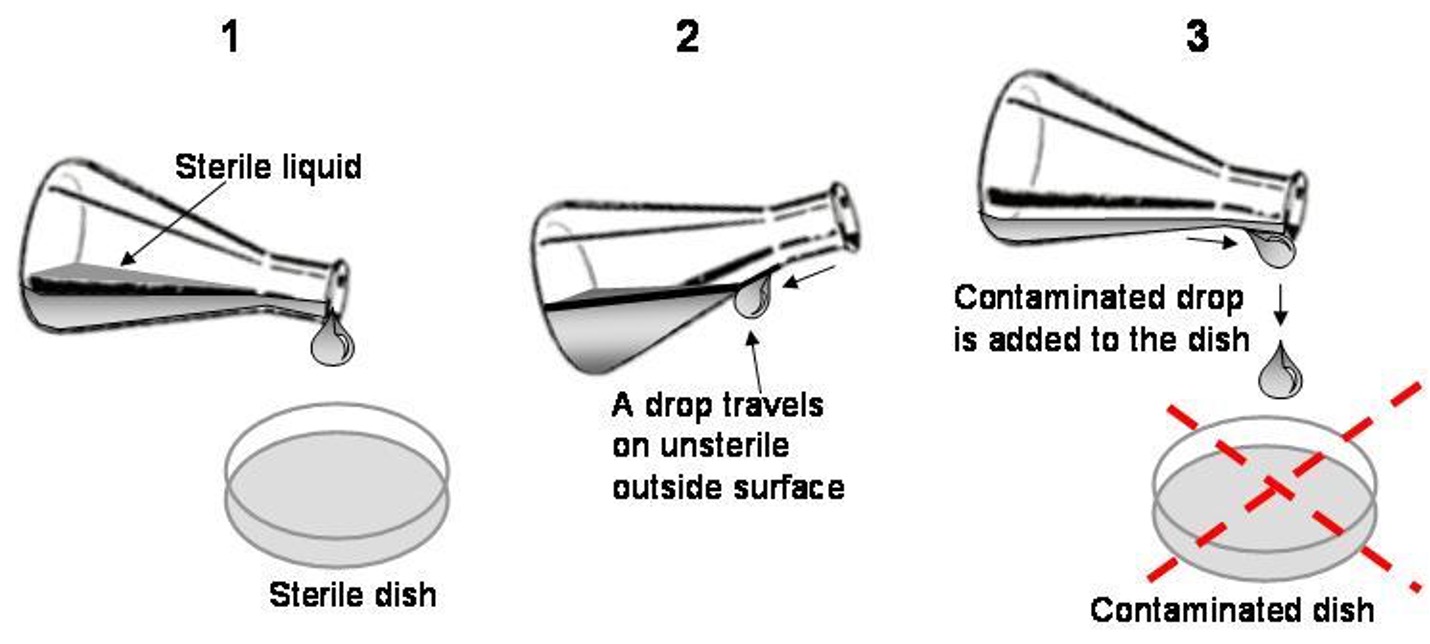
how contamination occurs
•Contact with non-sterile media, tips, pipettes, etc.
•
Non-sterile = open outside of the safety cabinet or
touched with a finger (even with the glove on!);
•Accidents or mistakes;
•Droplets of culture media fallout during manipulations or transportation of cell cultures;
•Crawling/growing into the culture vessels.
preventitive measures
ØKeep the lab (and lab coats!) clean,
ØIncubators and safety cabinets to be cleaned frequently (ethanol, virkon, UV)
ØAlways wear gloves and spray with 70% alcohol
ØAll items to be sprayed before placing them into the safety cabinet,
ØNever open media and other solutions outside of safety cabinet,
ØMonitor cell growth and routinely monitor for possible contaminations,
ØContaminated cultures – remove immediately!
ØCarefully with primary cells, isolate them from established cell lines,
ØAlways test new cell vials,
ØUse disposable, sterilized supplies whenever possible
growing cells in culture pros
–Use of animals reduced;
–Cells from one cell line are homogenous - same growth requirements;
–In vitro models allow for control of the extracellular environment;
–Allow to monitor various elements and secretions without interference from other biological molecules that occurs in vivo;
–Easier interpretation of experiment results.
growing cells in culture cons
–Must be carried out under strict aseptic conditions;
–
–Removal of cells from their in vivo environment means removing the cells, hormones, support structures and various other chemicals that the cells interact with in vivo;
–
–It is nearly impossible to recreate the in vivo environment. The artificial conditions may cause cells to de-differentiate which will cause them to behave differently and produce proteins other than it would in vivo.
Cell Culture in Research - used to study
•Gene/protein function
•Biological mechanisms of disease
•Signalling pathways
•Effects of molecules & compounds
Mechanism of action of drugs & toxins
Cell Culture in Research - interventions
•Transfections – adding DNA/RNA pieces encapsulated by liposome-like vesicles (fusion with cell membrane) OR viral particles packed with DNA of interest
•Treatments – adding to culture medium
- hormones, cytokines, neurotransmitters, toxins, drugs, inhibitors
HeLa Cells – used for studying:
Cancer
Parkinson’s Disease
AIDS
Polio, influenza vaccines
Haemophilia
Effects of radiation
Some genetic diseases
Drug analysis
industrial applications
•Toxicology
•Drug development and testing
•Cosmetics testing
•Disease modelling
•Recombinant proteins production
•Antibodies production
•Vaccines production
Cell therapy (e.g. CAR-T cells
primary cells vs cell lines
Characteristics | Primary cells | Established cell lines |
Lifespan | Limited - Hayflick Limit! | Infinite |
Growth requirements | Finicky, difficult to maintain in culture | Easy to maintain in culture; grow faster |
Closer to in vivo | YES; isolated directly from tissues | NO; clonally selected over time |
Modifications/mutations | LOW / NONE | Potentially HIGH |
Availability of donor data | YES | YES / NO |
Easy to obtain (in-house) | NO; technical hassle and animal handling/killing; | YES; can be obtained from a frozen stock |
why measure cytotoxicity
A) Integral part of drug discovery process:
Øto screen for cytotoxicity in compound libraries;
Øto screen potential drugs for unwanted cytotoxic effects before investing in their development as a pharmaceutical.
B) to predict response to treatment by testing primary cells from a patient (e.g. cancer cells from a tumour).
main types of assays
üMembrane integrity (e.g. release of an enzyme to culture medium*) = cytotoxicity tests.
Functionality tests (e.g. mitochondrial activity or ATP content in cells) = viability tests.- Can also be used to measure proliferation;
Functionality tests general procedure
Plate cells onto 96-well plates, leave to adhere
- treat the next day
Add drug to culture medium (several doses) and incubate for desired time
Add MTT or other reagent
Analyze the effect on cells
Repeat 3-5 times à statistical analysisPlate cells onto 96-well

Functionality tests general procedure advantages
96 well plates allow testing of several drugs and concentrations at the same time;
Quick analysis in a plate reader (absorbance or optical density, OD);
Different cell viability = different absorbance.
No need for elaborate cell counting using Trypan Blue
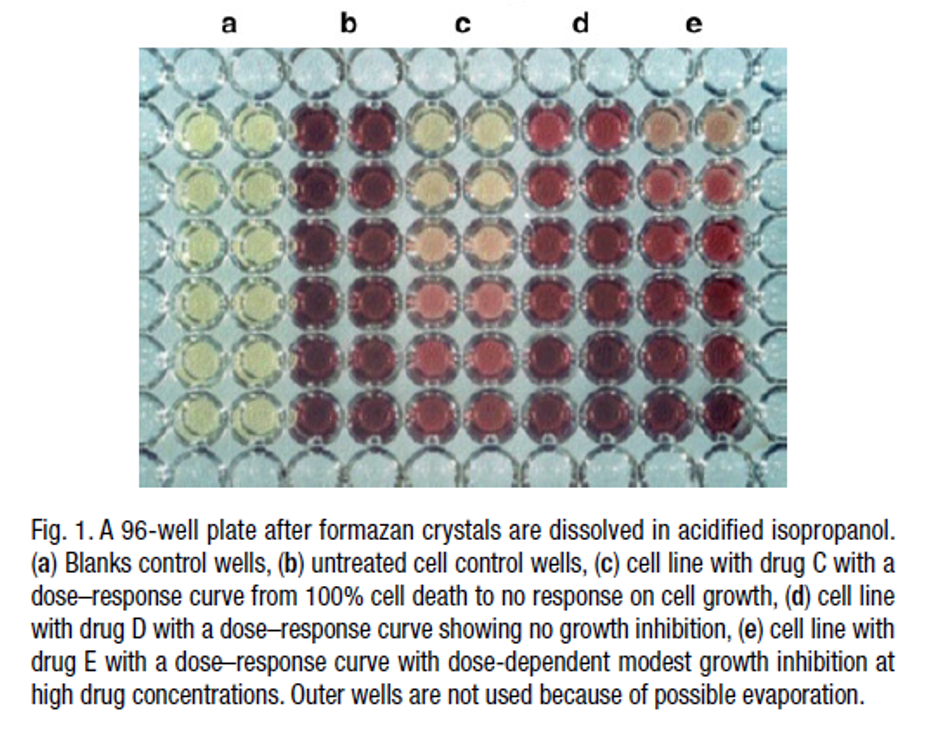
MTT assay - principle
at the end of treatment we add MTT to the wells.
MTT is converted to purple formazan by succinate dehydrogenase within the mitochondria of LIVE cells only.
mitochondrial activity of the cells in each well is reflected by the extent of conversion of MTT.
MTT strength of effect depneds on 3
Time of incubation (24h, 48h, 72h or 96h)
Drug concentration (nM, mM or mM)
Cell type.
For every experiment we need a control = cells without a drug (100% viability).
Always compare OD for cells treated with the drug to control OD.
MTT interpretations
If OD values of the treated cells are higher than control à increase in cell proliferation.
If OD values of the treated cells are lower than control à reduction in overall cell viability
- slower proliferation?
- cell death?