2. Crystallins, cataract, collagens, sclera
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
44 Terms
what are crystallins?
-water soluble protein
-90% protein in lens
-increases index of refraction in lens, maintains transparency by having soluble, high molecular weight aggregates
crystallins are similar to what proteins?
heat shock proteins (α-crystallins) and a bacterial spore coat protein (β and γ-crystallins)
where are crystallins found?
found in heart, brain and lungs as well as in the cornea of the eye
-role in lens in structural
3 types of crystallins?
alpha 35%, beta 55% , gamma 10%
alpha crystallin characteristics
-largest
-a-helical and B-sheets
-N terminus acetylated
-possibly globular
-quaternary structure uncertain
-least amount of cysteines
-small heat shock protein (sHSP)
-chaperone activity
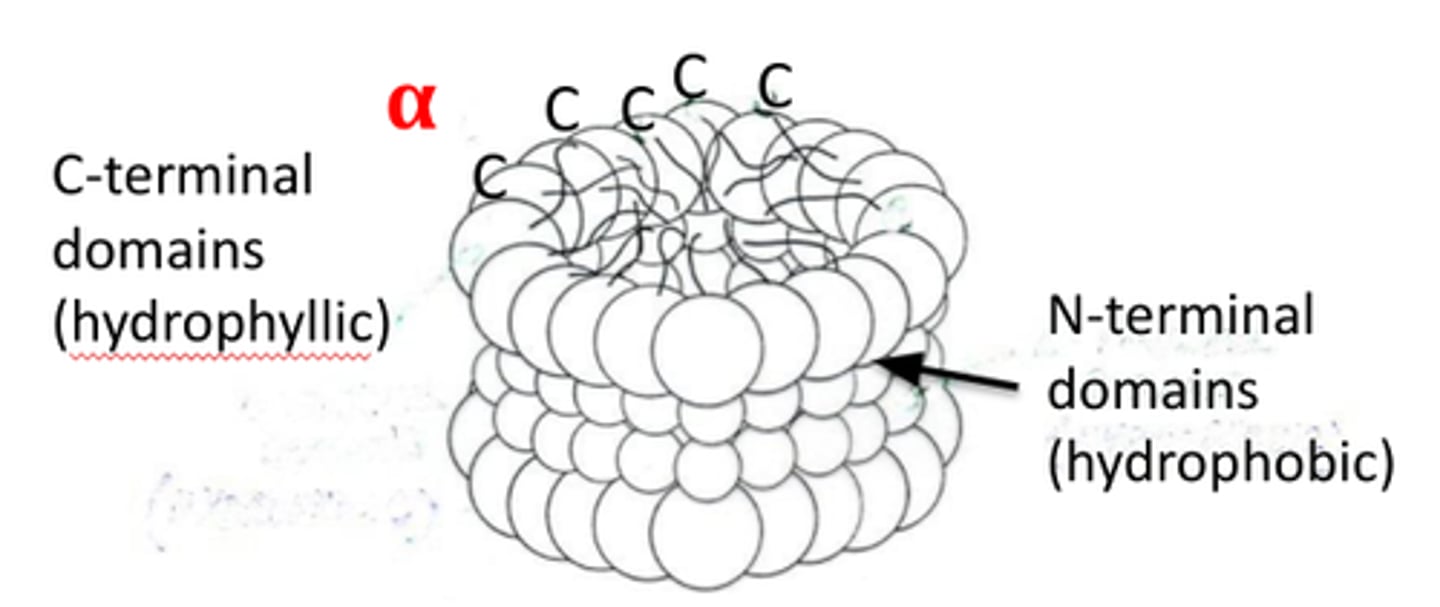
what crystallins are in the lens epithelium?
alpha-crystallins
Beta crystallin characteristics
-second largest
-just B-sheets
-N terminus acetylated
-probably globular
-2nd largest amount of cysteine
-quaternary structure is a large globe of polypeptides
gamma crystallin characteristics
-smallest
-N-terminus not acetylated
-just B-sheets
-globular tertiary structure
-no quaternary structure (single polypeptide)
-most cysteines
acetylation of crystallin structures
-in alpha and beta crystallin
-addition of acetyl group to N-terminus
-prevents against protein degradation
phosphorylation of cystallin structures
addition of phosphate group to serines on alpha-crystallins (or threonine on other proteins)
-adds (-) charges
-weakens the chaperone properties of α-crystallins by opening its structure
glycosylation of crystallin structures
addition of sugars to serines on alpha and beta crystallin
deamidation of crystallin structures
-conversion of Asparagine to Aspartic acid and Glutamine to Glutamic acid on α and β crystallins.
-Could be part of aging process
oxidation of crystallin structures
conversion of S-H to S-S disulfide or
SOCH3 to S-CH3 on alpha and beta crystallins
-could be part of aging process
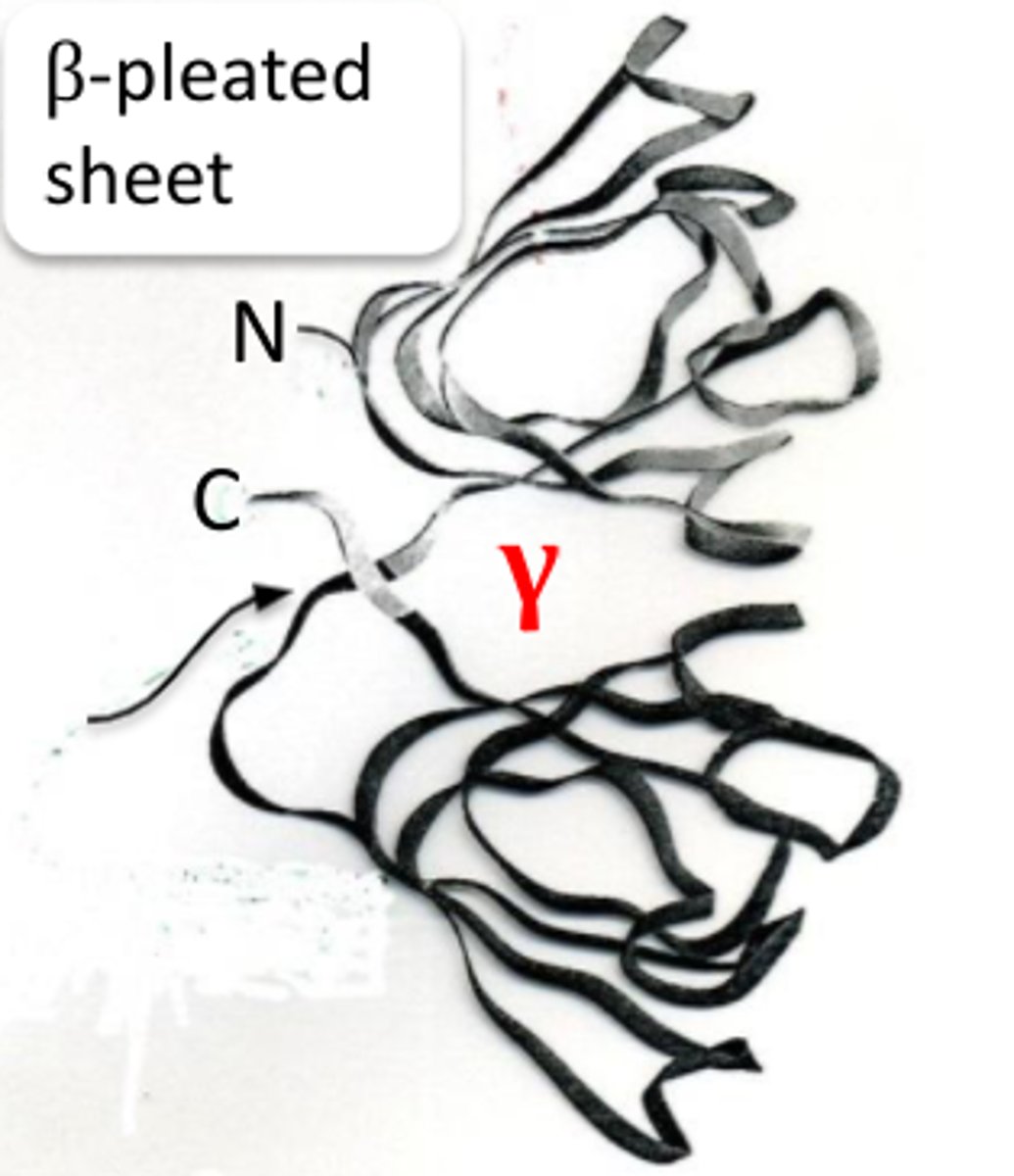
simplest crystallin structures
gamma crystallin
-no quaternary structure 1 polypeptide
-They consist of a sets of beta pleated sheets joined by a connecting peptide
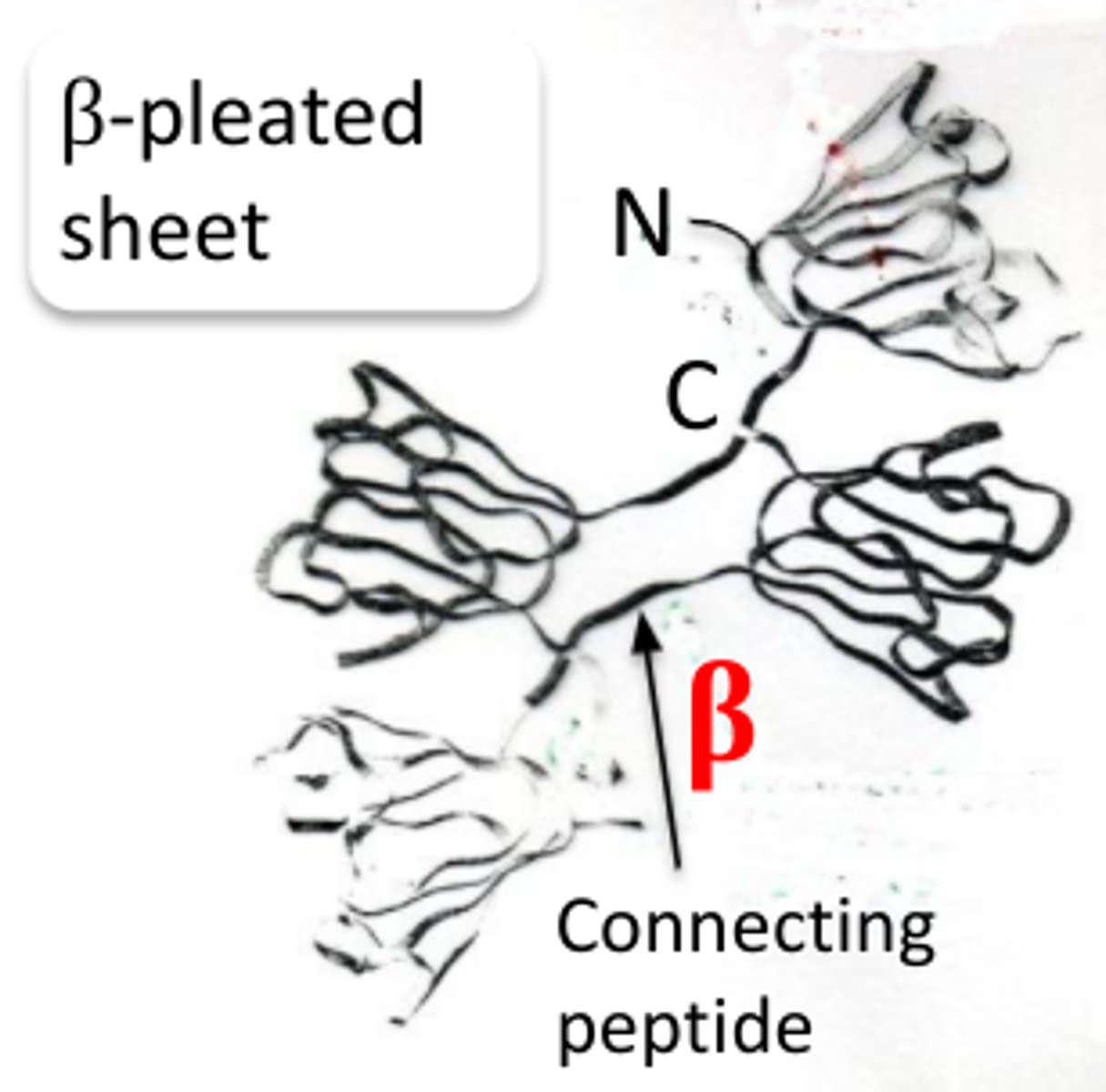
Beta crystallin structure
-consist of at least four structures of beta pleated sheets in
which there are two n-terminal ends and two c-terminal ends;
-there are two polypeptide chains.
-aromatic and sulfur rich residues
alpha-crystallin structure
triple hybrid structure
-alpha helix and beta sheets
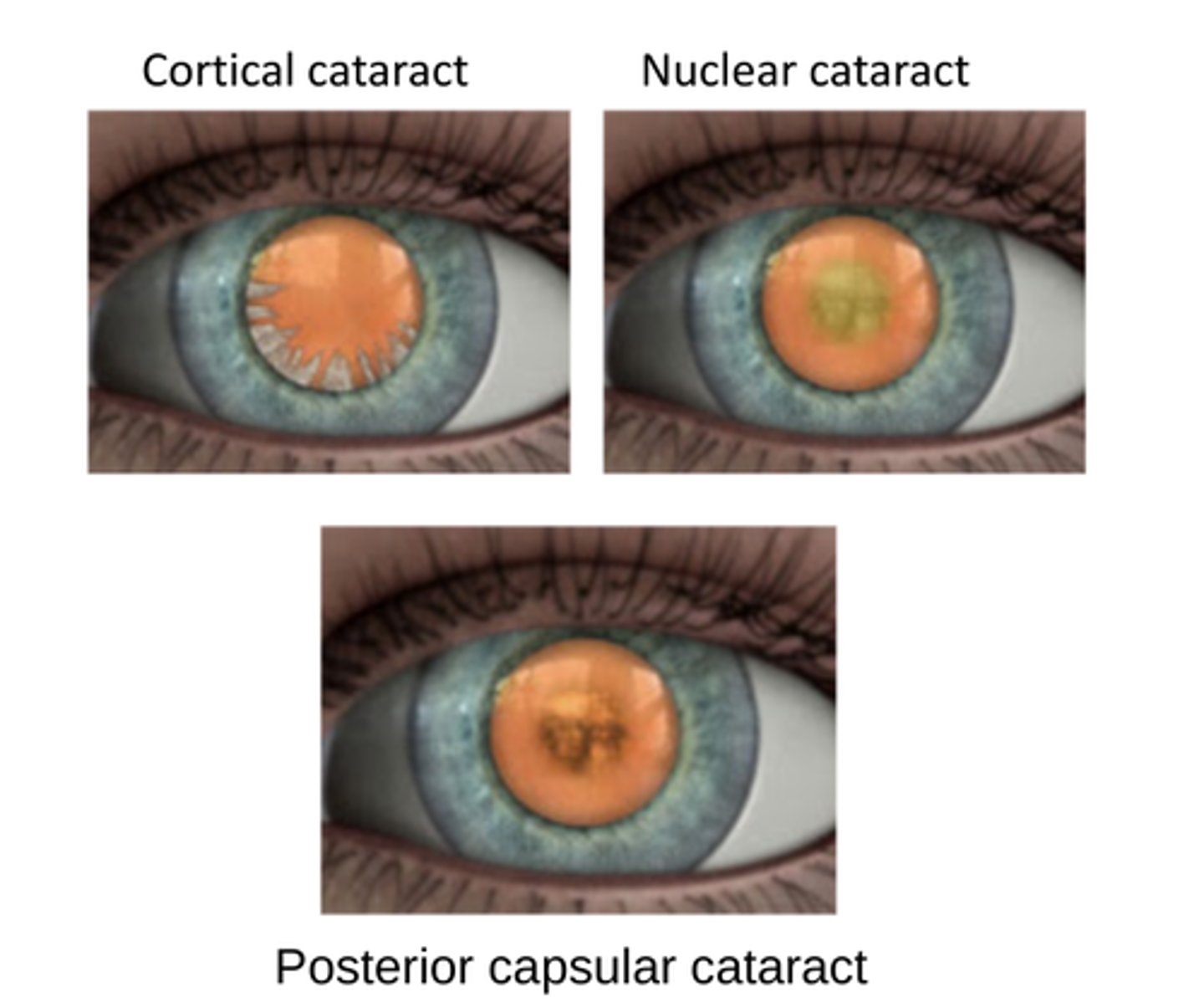
cataracts
-lens opacification, results from aggregation of crystallins
-light scattering due to an accumulation of aggregates greater than 1000 angstroms in size.
-may be gradual or sudden with opacities in both the nucleus (inner region) and/or the cortex (outer region) of the lens.
-senile (age related)
how do lens epithelial cells become lens fiber cells?
lens epithelial cell loses its proteins and nucleus
loses water content
goes from 15% protein to 90%
depends on crystallin for structural support
what promotes cataract formation? how does lens combat this?
Post-translational modifications of proteins seem to promote cataract formation (with age), oxidation, radicals, chemical, diabetes, hereditary
-This can be prevented by the chaperone activity of α-crystallin
Hydrogen peroxide (H2O2) is present in the aqueous and causes oxidation. How does the lens protect itself?
anti-oxidative activity of glutathione
ex: Glutathione becomes oxidized instead of crystallin
The lens is subject to UV radiation, which produces free radicals. How does the lens protect itself?
high concentration of Vitamin C (ascorbate) in the aqueous.
What are the damaging effects of Post-translational modification in crystallins
-weaken the chaperone properties of α-crystallins by opening its structure (negative charges of phosphate groups)
-make all crystallins subject to oxidation (disulfide bond formation) and the beginning of protein complex formation
-free radicals are atoms with unpaired electrons that readily react with proteins and lipids
-hydrogen peroxide causes similar effects on proteins and lipids
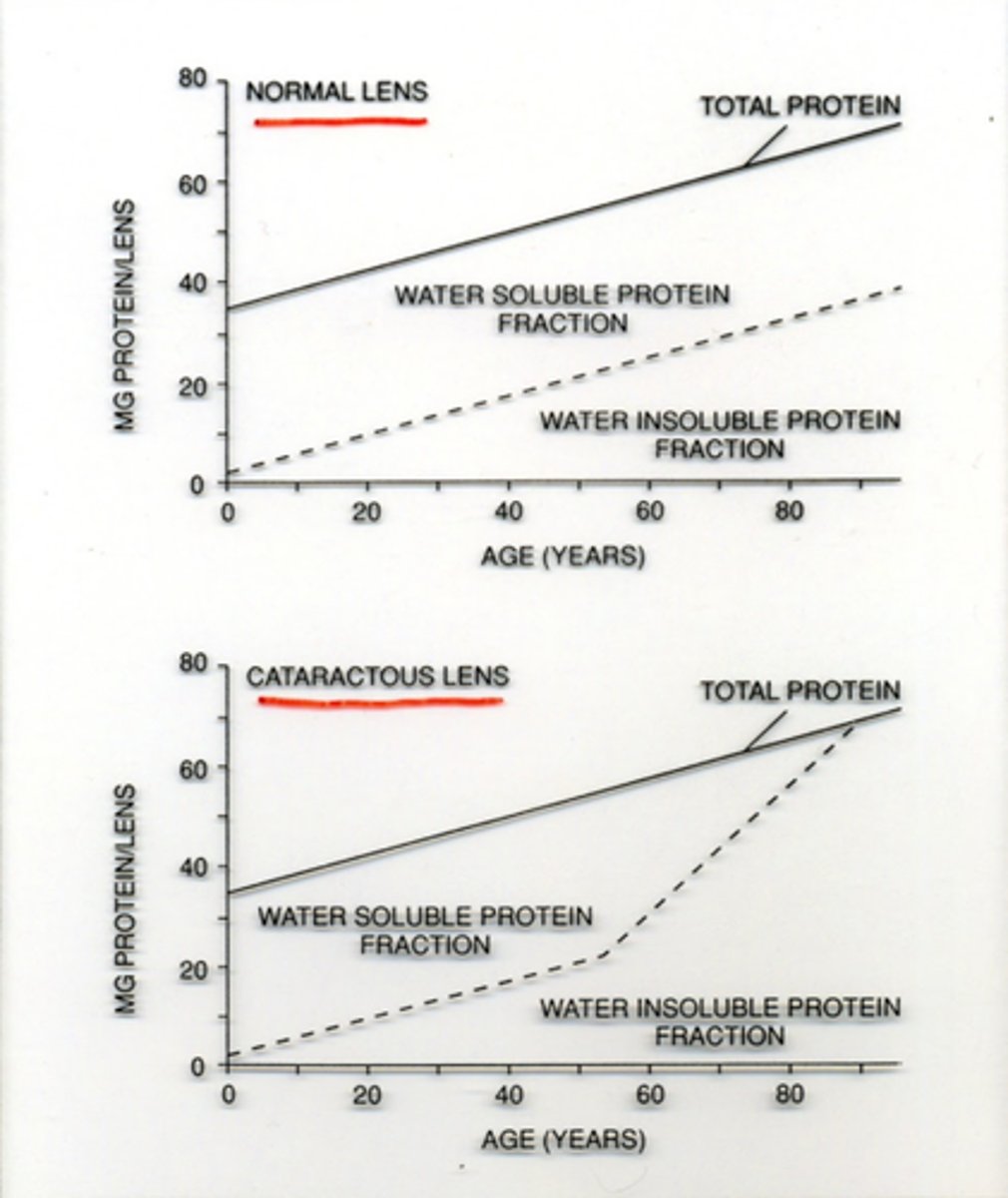
What happens to the lens as we age?
develop more insoluble proteins
what is a difficult cataract for patients?
Nuclear cataracts are more difficult for the patient since they both scatter light and absorb light causing an increased disruption in vision.
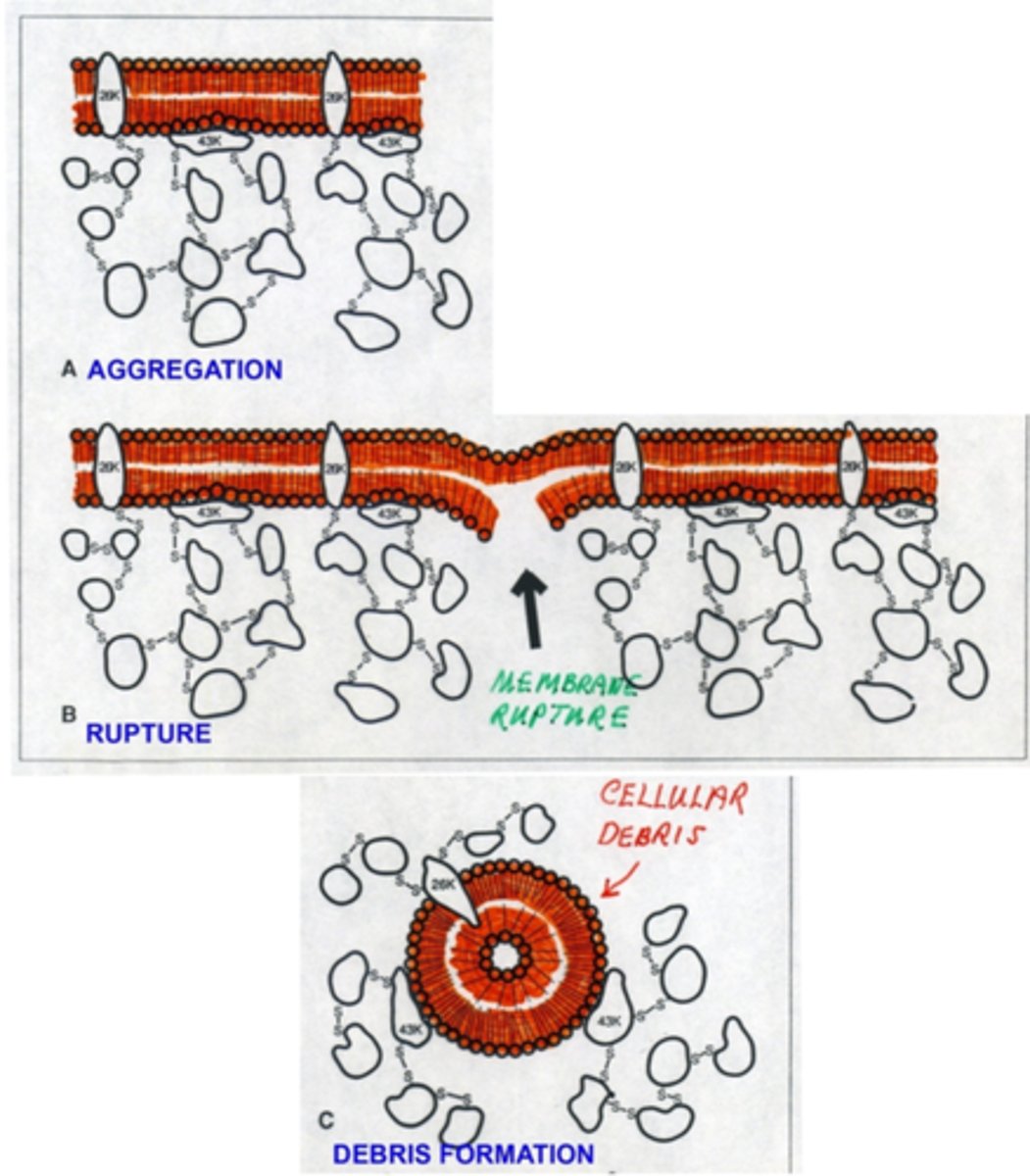
mechanisms for cortical cataract formation
1. aggregation of crystallins to intrinsic 26kd and extrinsic 43 kd membrane proteins
2. rupture of membrane
3. debris formation
collagen characteristics
-insoluble
-proline- Hydroxyproline-Glycine
-3 left handed helices wrapped into a right handed supercoil triple helix
3 main classifications of collagen
Class I, II, III
-refers to the structure of collagen
Describe the structure of class I collagen
Banded, fibrillar collagens. They
exhibit long uninterrupted domains
Describe the structure of Class II of collagen
-Contain interrupted triple helices and include "FACIT" collagens (Fiber Associated Collagens with Interrupted Triple Helices)
Describe the structure of Class III Collagen
Not attached to fibers such as anchoring rods
Describe Type I collagen
is the most abundant in the human body, found in skin, tendons, vascular tissue, organs and bones.
-Gram for gram, type I collagen is stronger than steel.
Describe Type II collagen
-is the basis for articular cartilage (dense connective tissue) and hyaline cartilage (“gristle”).
-provides tensile strength to the tissue.
Describe Type III collagen
-is a fibrous scleroprotein that is often found alongside type-I collagen.
-Scleroprotein is a simple protein found in horny and cartilaginous tissues, and in the lens of the eye.
Describe Type IV collagen
-The C-terminus domain is not removed during post-translational modifications and so the fibers link head to head rather than in parallel.
-This type also lacks the regular glycine in every third residue so the helix structure is not tight and exact, causing it to form a sheet: the basal lamina
T/F The collagen superfamily has at least 28 collagen types.
T
-type refers to the molecular structure of collagen
what collagen makes up the sclera and cornea?
type 1 collagen
corneal stroma is made of collagen lamellae. They have no limiting membrane. How are they connected to each other?
1. disulfide bonds (fiber to fiber)
2. Fibers that cross over and weave into the fibers of adjacent lamellae
3. Lamellae that often divide into smaller components that later merge with adjoining lamellae
4. Sometimes lamellae split into two to three sub-layers that become interwoven
does cornea contain the GAG hyaluronic acid?
no, some at end of limbus and increases into sclera & vitreous
The cornea regulates the size of its type I collagen fibers via type ? collagen fibers
V, the more V fibers, smaller the collagen I fiber
-Type V fibers are acidic at their N-termini.
-This, or possibly steric hindrance, may inhibit new type I fibers.
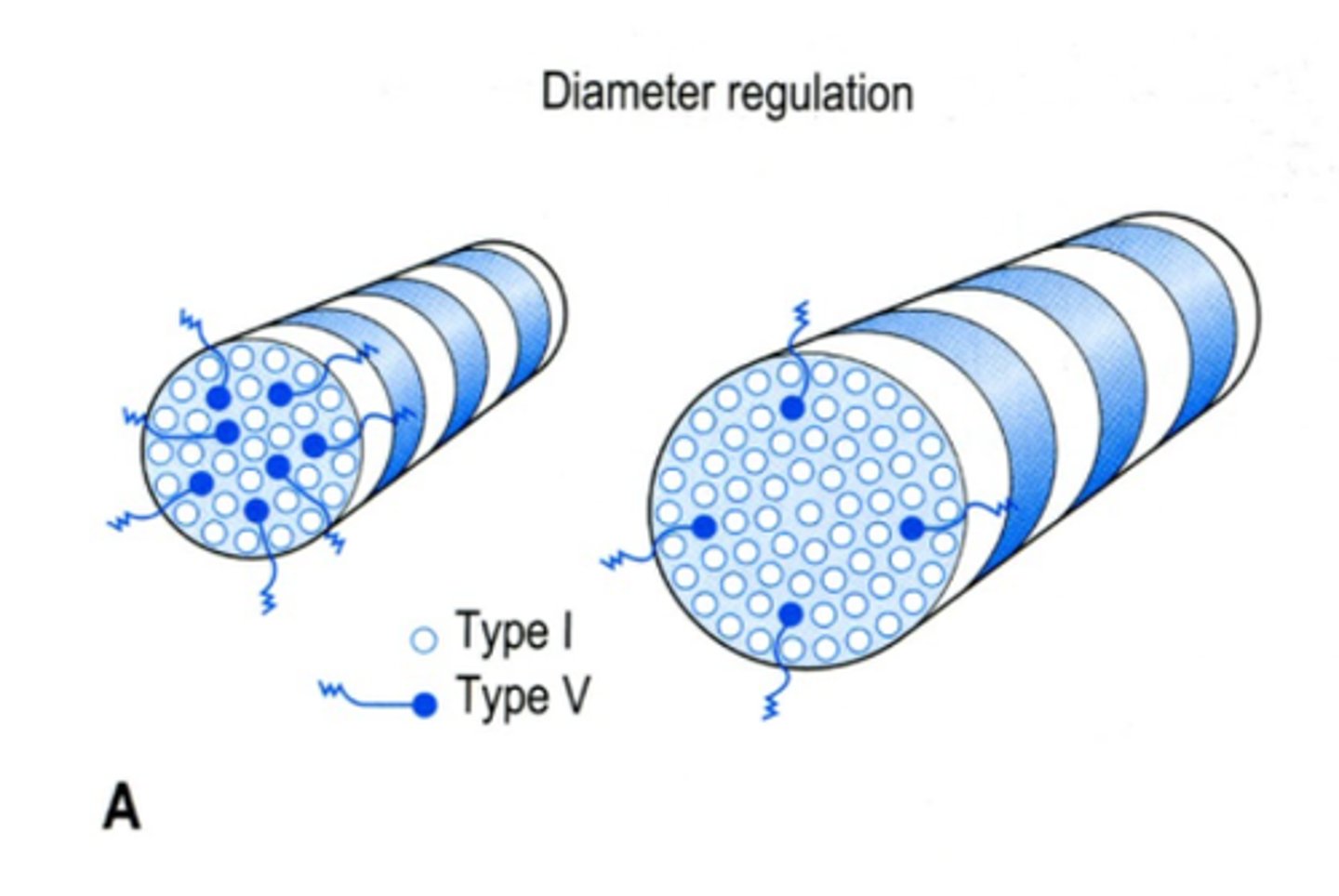
How is corneal transparency maintained?
by equidistant fiber spacing ...this is accomplished by mutual interference and cancellation of light scattering.
-proteoglycans (PGs) (proteins +GAGs) bound to V collagen fibers
-unfortunately their negative charges can cause stroma swelling
what collagen makes up bowman's layer?
made up of a condensation of types I and VII (7) together with type III.
1, 3, 7
what collagen makes up descemets membrane
Descemet's membrane contains types V, VIII, IX and XII
5,8,9,12
-special lattice structure
How is collagen arranged in vitreous?
Type 2 big collagen fibers surrounded by smaller hybrid type IX/XI FACIT collagen fibrils.
spaghetti hyaluronate GAGs in spaces
-NC4 on FACIT collagen is considered to be the non-collagenous area that associates with GAG hyaluronate.

How does hyaluronate in vitreous contribute to elasticity?
hyaluronate contains large volumes of water and swells between the two components IX/XI fibrils and type II collagen fibers. This gel is both elastic and readily transmits the IOP.