Chemistry Honors
5.0(1)
5.0(1)
Card Sorting
1/35
Earn XP
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
1
New cards
Element or Compound
Pure substance (homogenous of one of these throughout material)
2
New cards
Heterogenous
Anything from animal is?
3
New cards
Homogenous
Air
4
New cards
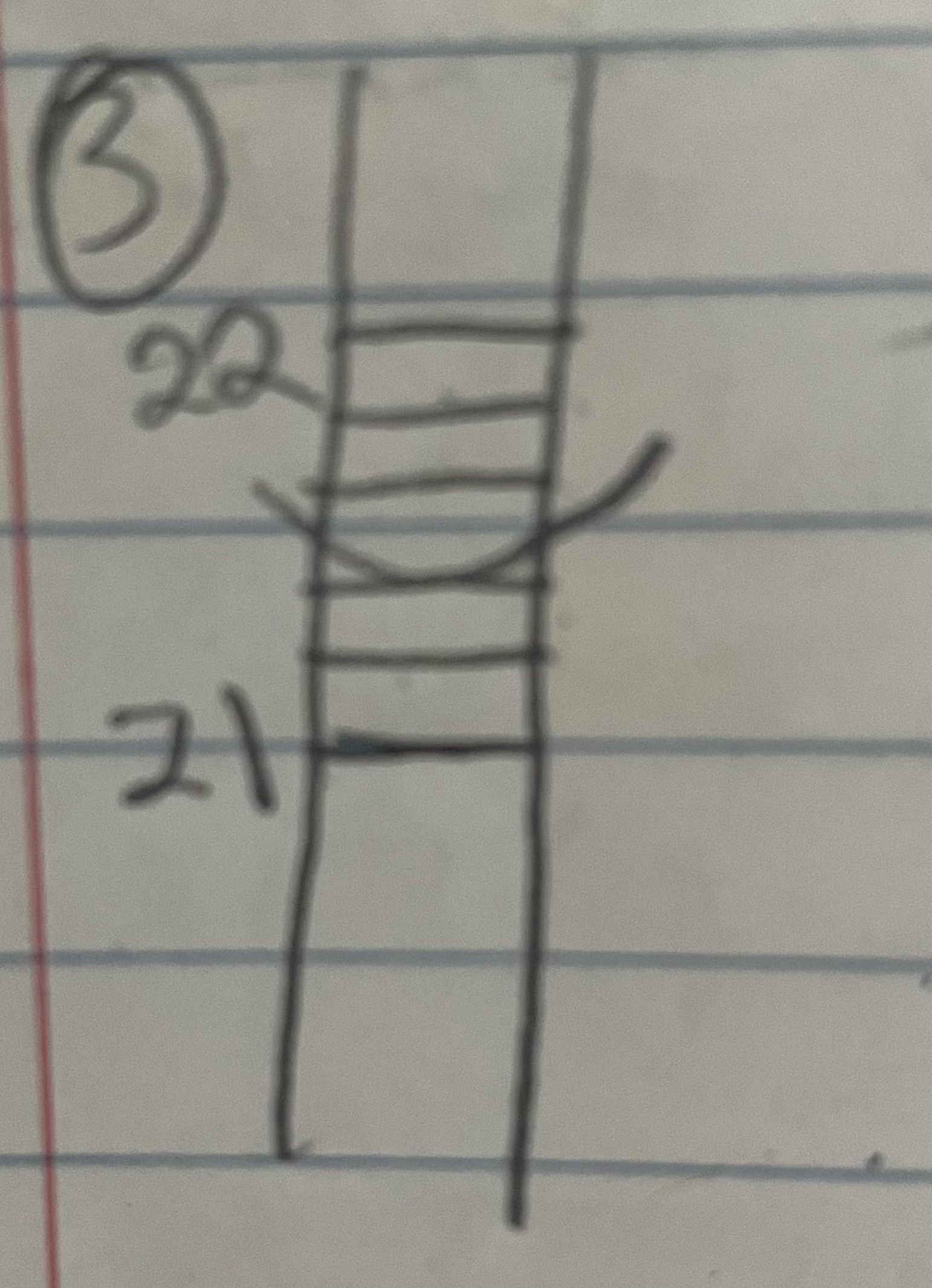
21\.40
What is the measure?
5
New cards
How close measurement is to true value
Accuracy
6
New cards
How close measurements are to each other
Precision
7
New cards
Change in identity of substance
Chemical change
8
New cards
Change is state of matter and dissolving
Physical change
9
New cards
Solid to gas
Sublimation
10
New cards
to deliver
TD
11
New cards
to contain
TC
12
New cards
Not accurate, boiling, stirring, estimation, reaction vessel
Beaker

13
New cards
Not accurate, swirling, filtration, titration
Erlenmeyer Flask

14
New cards
accurate (TD), everyday measurment
Graduated cylinder

15
New cards
Very accurate (TD), small amounts
Glass pipet

16
New cards
Very accurate (TD), larger amounts, titration
Buret

17
New cards
Very accurate (TC), making a solution of certain concentration, meant to measure only specific amount
Volumetric Flask

18
New cards
9 zeroes
Giga (G)
19
New cards
6 zeroes
Mega (M)
20
New cards
3 zeroes
Kilo (K)
21
New cards
2 zeroes
hecto (h)
22
New cards
1 zeroes
deka (da)
23
New cards
0 zeroes
Unit
24
New cards
1 zero
deci (d)
25
New cards
2 zeroes
centi (c)
26
New cards
3 zeroes
milli (m)
27
New cards
6 zeroes
micro (µ)
28
New cards
9 zeroes
nano (n)
29
New cards
1g/1mL
Density of water
30
New cards
Cubed
Length Units that need to be volume
31
New cards
1 g = 1 mL
What is 1 gram equal to in mL?
32
New cards
1 in = 2.54 cm
What is 1 inch equal to in centimeters?
33
New cards
1 mL = 1 cm^3
What is 1 mL equal to in centimeters?
34
New cards
1 gal = 3.79 L
What is one gallon equal to in liters?
35
New cards
1 lb = 454 g
What is 1 pound equal to in grams?
36
New cards
1 lb = 16 oz
What is one pound in ounces?