Electron Configurations and the Bohr Model in CHM 1310
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
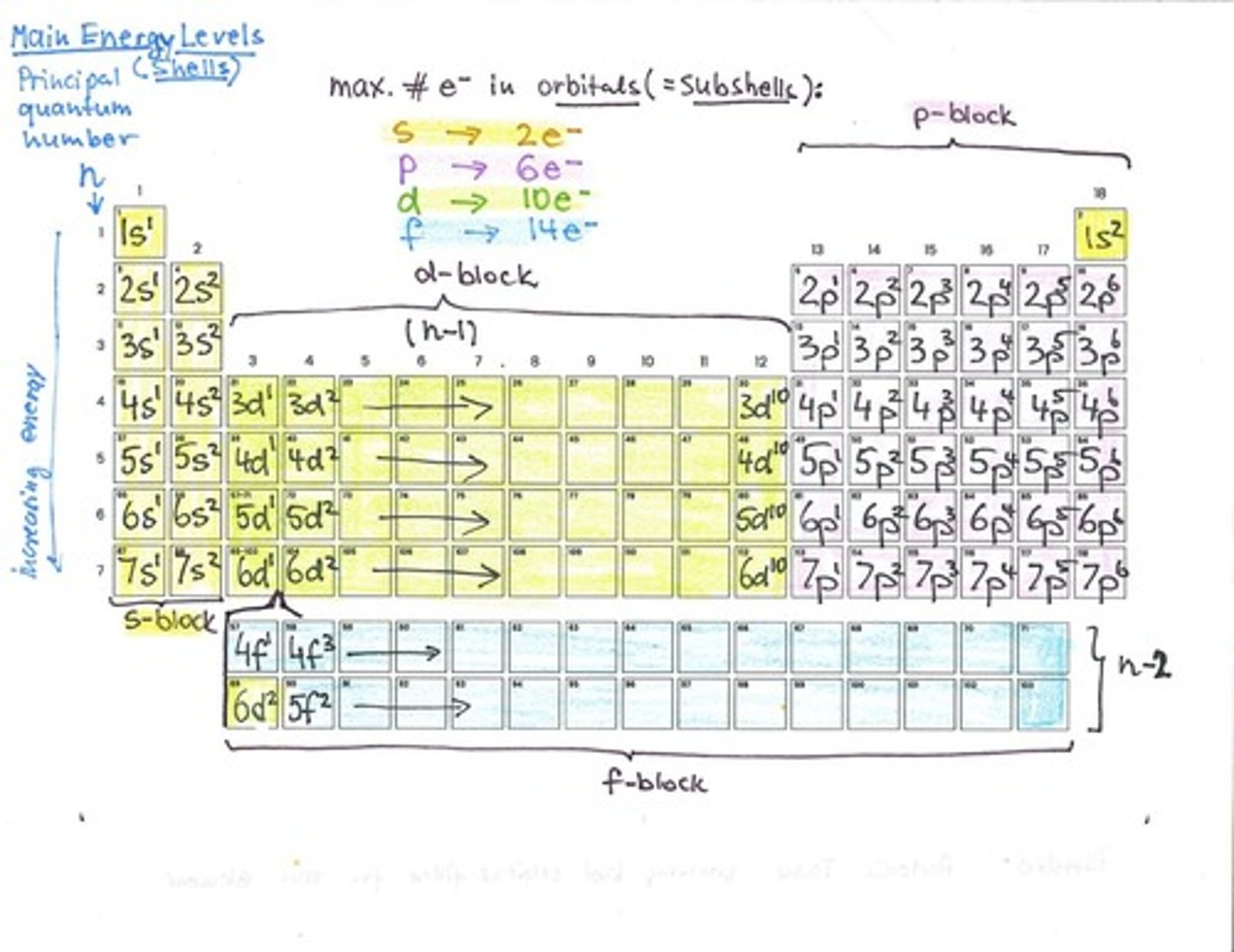
Principal Quantum Number (n)
Indicates the energy level of an electron.
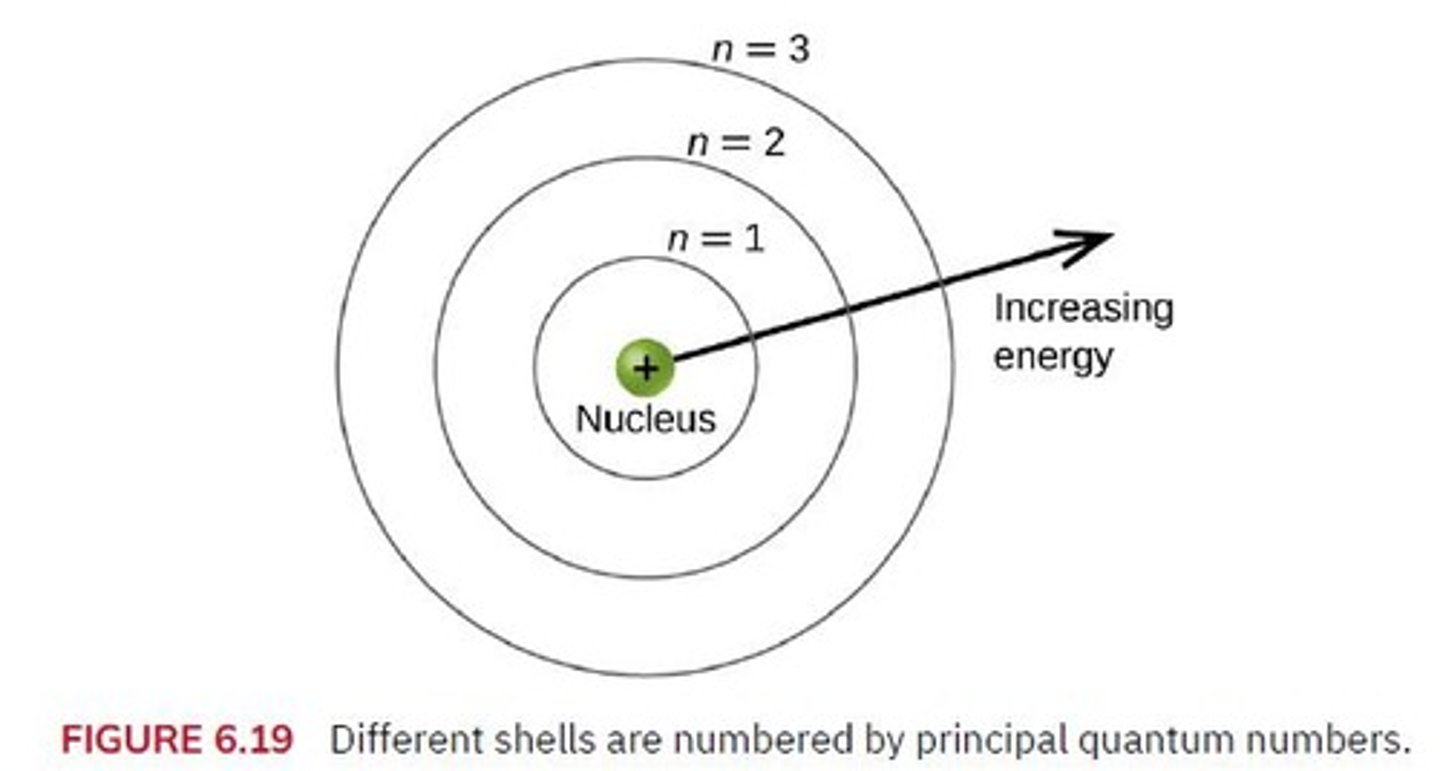
Bohr Model
Outdated model of atomic structure.
s Orbital
Spherical shape, holds up to 2 electrons.
p Orbital
Dumbbell shaped, holds up to 6 electrons.
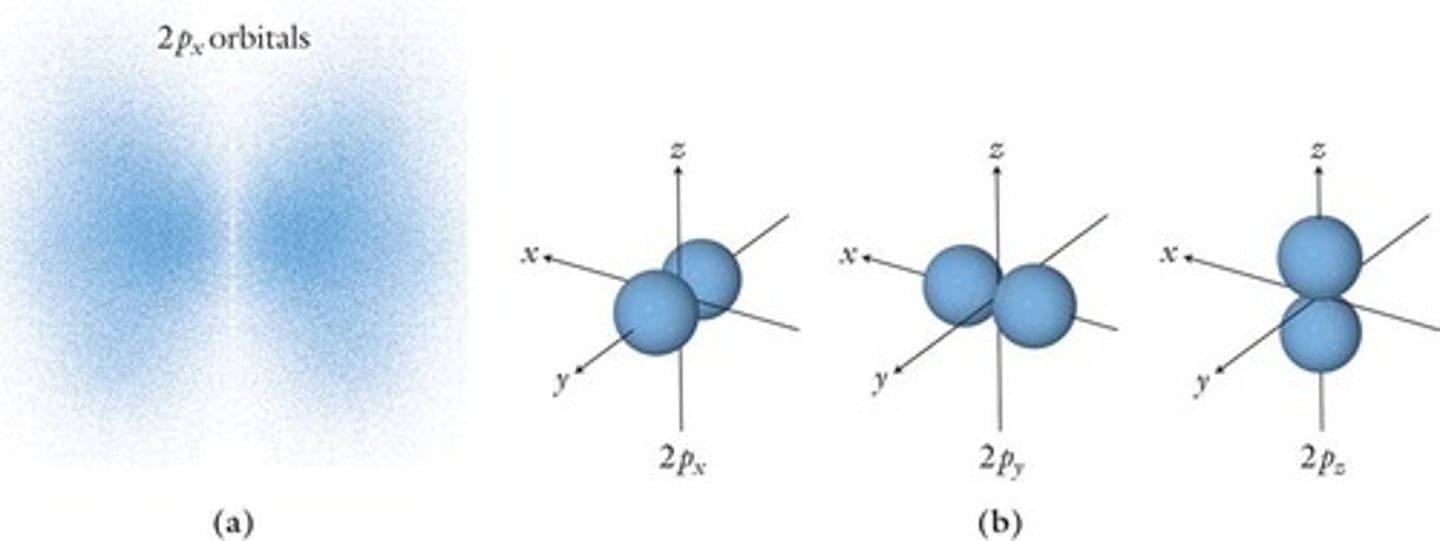
Electron Orbitals
Regions defining electron probability locations.
Maximum Electrons in Orbitals
s: 2, p: 6, d: 10.
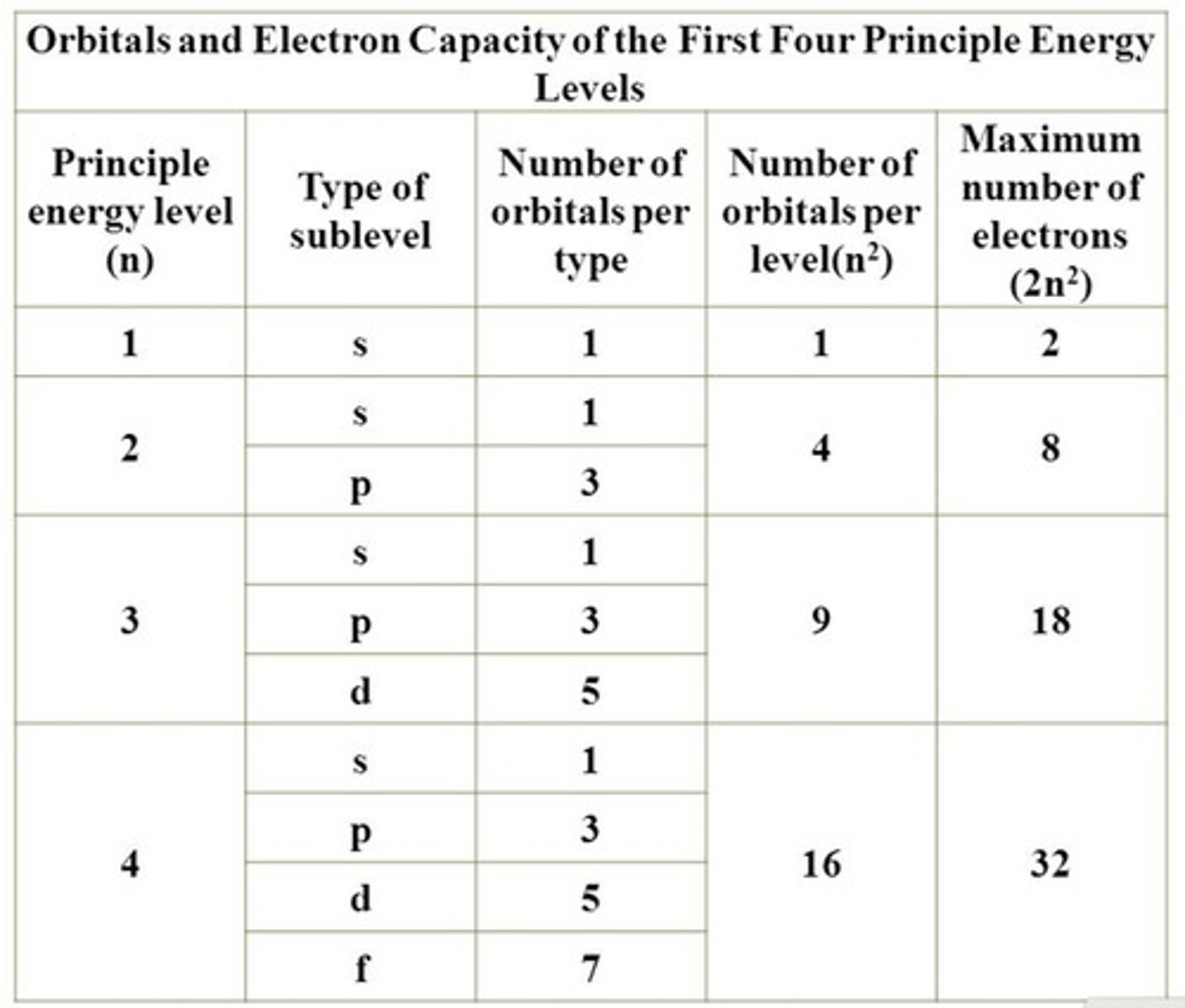
Sublevels/Subshells
Types of orbitals: s, p, d, f.
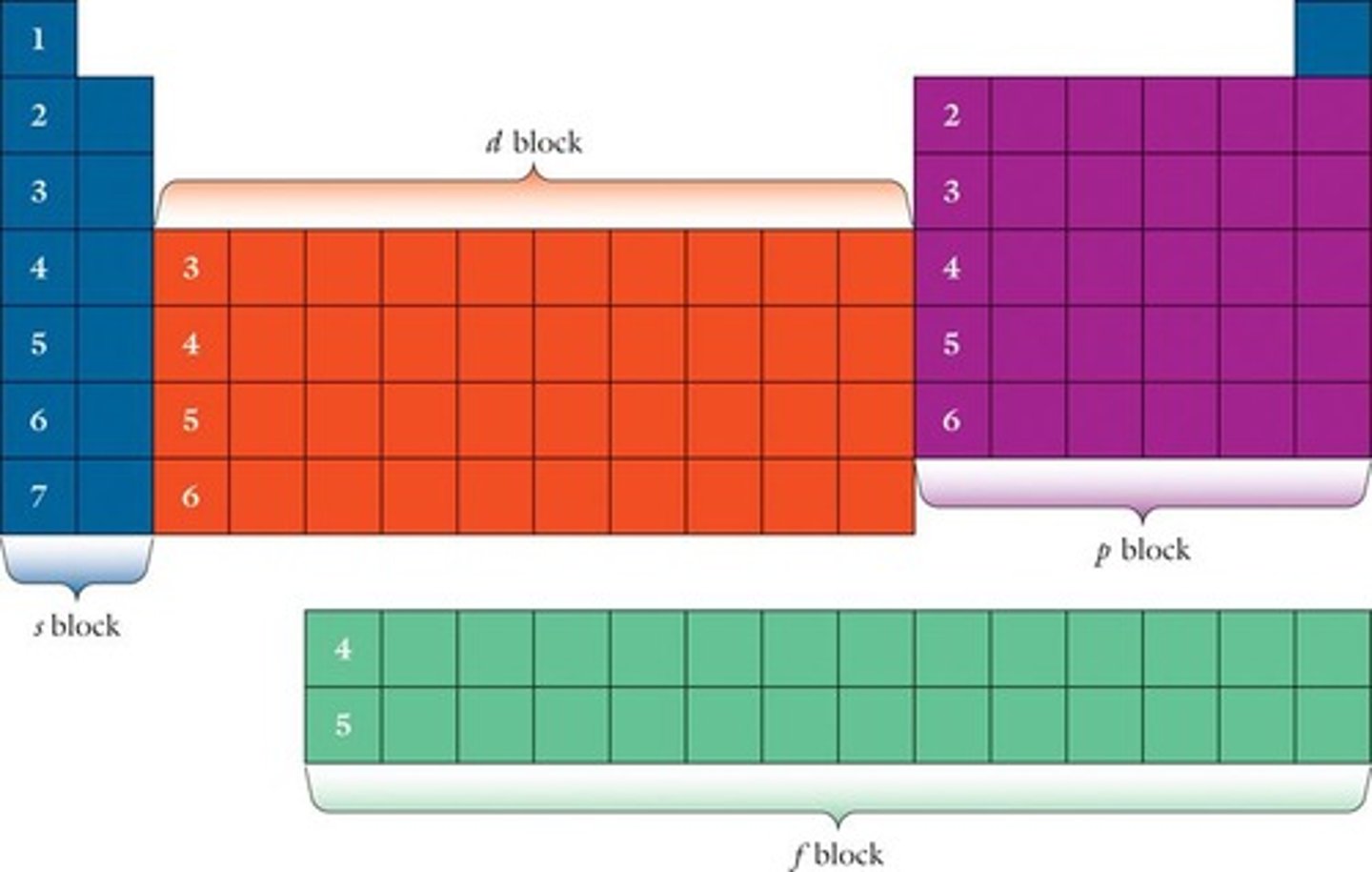
Electron Configuration
Distribution of electrons among orbitals.
Beryllium Electron Configuration
1s²2s², total 4 electrons.
Noble Gas Configuration
Shorthand notation using previous noble gas.
Probability in Orbitals
Describes likelihood of finding electrons.
Electron Excitation
Electrons moving to higher energy levels.
Energy Level
Main shell where electrons reside.
Electron Filling Order
Orbitals filled in order of energy.
Node in p Orbital
Region where probability of finding electron is low.
Transition Metals
Exceptions in determining principal quantum number.
Periodic Table Rows
Correspond to principal quantum number n.