Passive Transport and Membrane Potentials
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
42 Terms
what are spontaneous processes driven by?
potential energy gradients
describe the spontaneous process of a hot cup of coffee that is sitting at room temperature (potential energy is going down, there is a potential heat energy difference/gradient)
as chocolate cools, the temperature gradient decreases/increases and the rate of cooling decreases/increases
When the potential energy gradient reaches 0, what happens?
energy (heat) flows from hotter liquid to cooler air
bigger the difference in temperature the fster the energy flows
as cup cools down, heat flow of energy from cup to air also slows down
surrounding air is warmed up a little by heat flow from hot coffee
as chocolate cools, the temperature gradient decreases and the rate of cooling decreases
When the potential energy gradient reaches 0, no further cooling occurs and the system reaches equilibrium
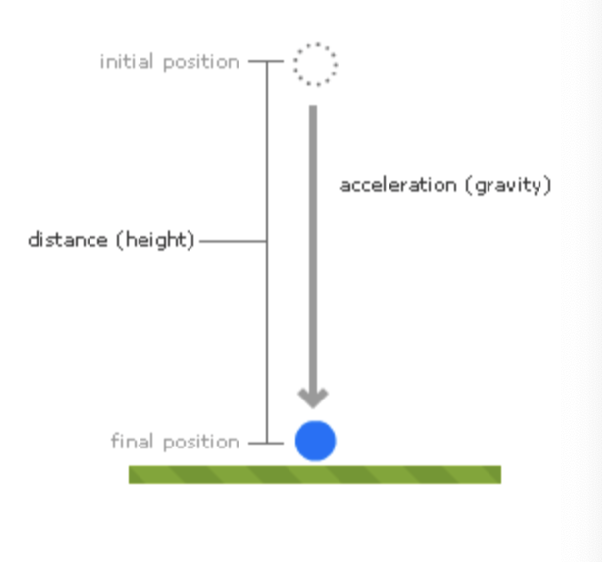
describe the potential energy gradient that exists in this system: a falling ball
what is the potential energy gradient in this example? what would equilibrium be defined as?
the potential energy gradient here is the difference in height, h, of the ball in the initial vs the final position
equilibrium is E = 0
many types of potential energy gradients exist including
thermal, pressure, electrical, gravitational, chemical, concentration, etc
in every spontaneous system, what are 3 main processes that occur?
potential energy graident exists initially
gradient goes to zero during the spontaneous process
the process stops when the gradient reaches 0
what is it called when solutes spontaneously move from a region of higher concentration to a region of lower concentration?
diffusion
describe the potential energy gradient that exists in this system: a sugar cube placed in a cup of coffee
• Initially all sugar is in the cube – highly concentrated
• sugar spontaneously disperses into the volume of fluid until it is evenly distributed = equilibrium
• Thus, concentration is another form of potential energy
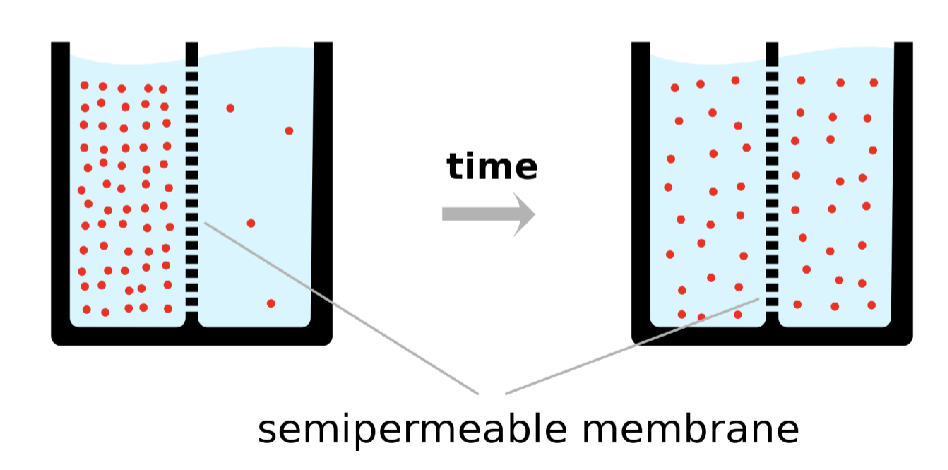
describe the potential energy gradient that exists in this system: diffusion across a membrane
most of the solute molecules (red dots) are initially located on left side of membrane
concentration gradient exists between the left and right side.
If membrane is permeable to the solute, it will move to the right side until the gradient goes to zero
what drives diffusion?
concentration or chemical potential gradient
the rate of diffusion decreases _____________
logarhythmically
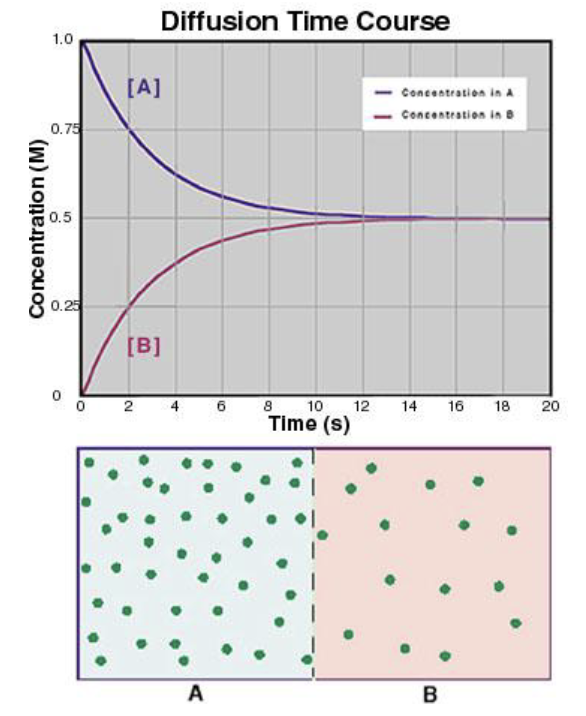
why does the rate of diffusion decrease logarhythmically?
because the solute concentration gradient decreases as diffusion occurs
what is the formula for finding equilibrium of simple diffusion?
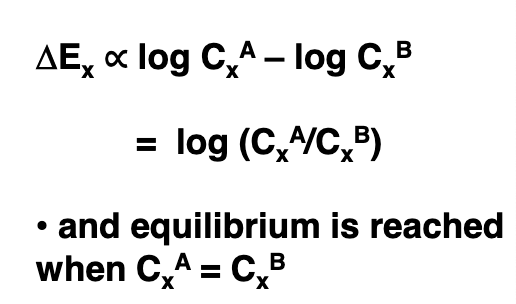
what is the law of electroneutrality?
ionic solutions must have equal numbers of positive and negative charges (solutions are neutral)
Ionic compounds dissociate in water into positive and negative ions. These are called…?
counter ions
Although the ions dissociate and become hydrated, they retain a strong attractive force for each other because of their opposite charges
KCl → K+ + Cl-
what condition places a constraint on diffusion of ions, particularly when a membrane is permeable to one ion but not its counter ion as is often the case?
law of electroneutrality: ionic solutions must have equal numbers of positive and negative charges (solutions are neutral)
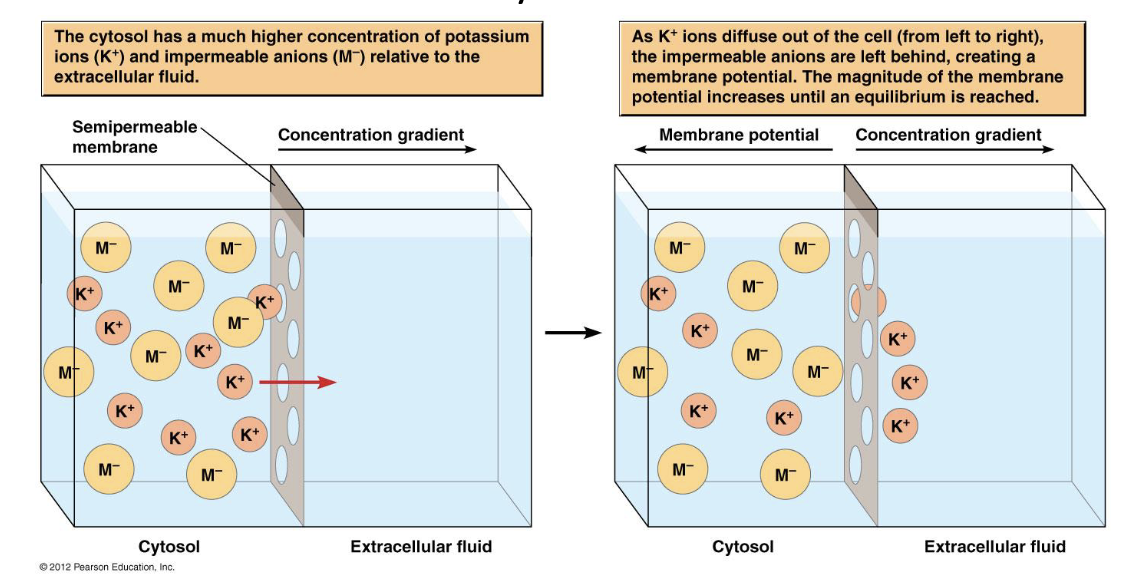
describe what’s happening in this diagram.
what happens to the membrane?
a membrane separates inside of cell (cytosol) from outside
cytosol contains K+ and anions (M-)
membrane is permeable to K+ but not M- ions
K+ ions diffuse from cytosol to extracellular surface of the membrane but cannot move farther because M- ions are not permeable and remain behind on the cytosolic side of the membrane.
The electrical attraction between K+ and M- keeps them associated in the vicinity of the membrane.
membrane becomes increasingly positive on outside and negative on inside
membrane becomes polar and gains an electrical potential
It becomes progressively harder for additional ions to accumulate on the membrane since the K+ ions already on the outside repel additional K+ ions and the M- ions repel additional M- ions
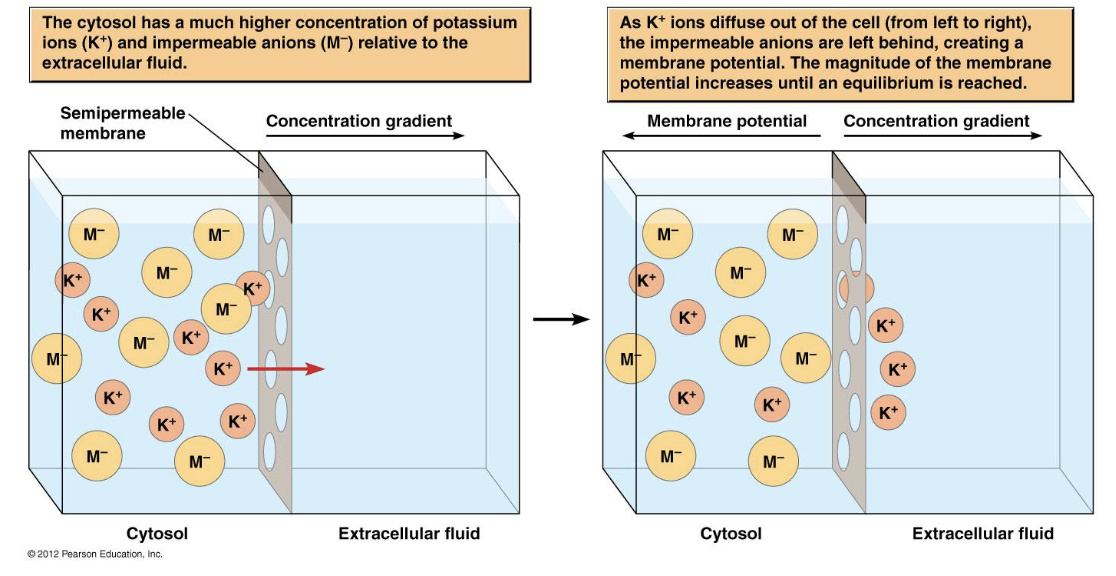
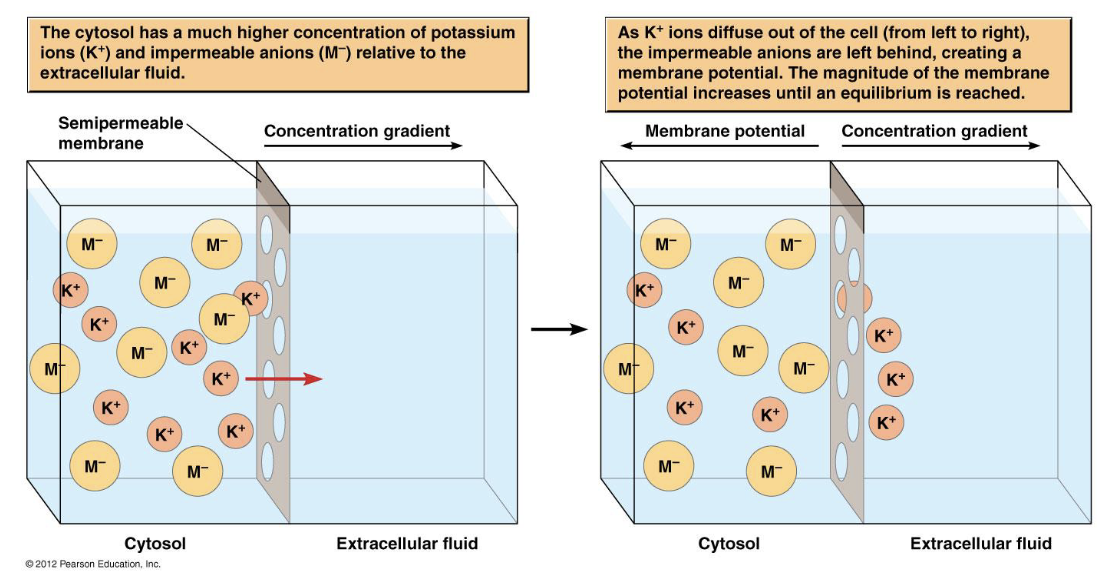
when is equilibrium reached in this situation?
when membrane potential in mV is great enough to stop diffusion of more K+
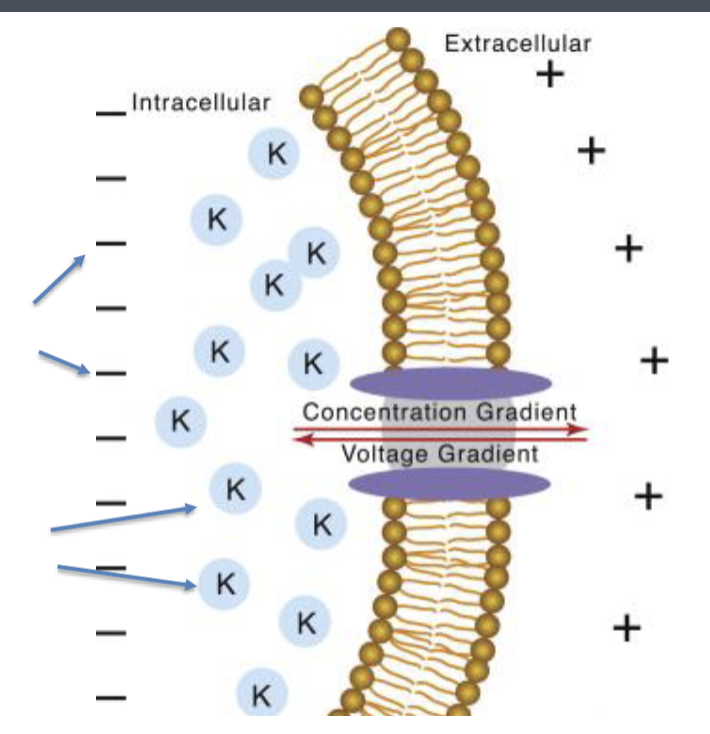
what is happening in this situation? when is equilibrium reached?
the higher ion concentration inside the cell drives K+ to the outside resulting in positive charge accumulating on the membrane
BUT
the negative counter ions (Cl-) that exist intracellularly create a voltage gradient that opposes the concentration gradient
equilibrium is reached when concentration gradient = voltage gradient
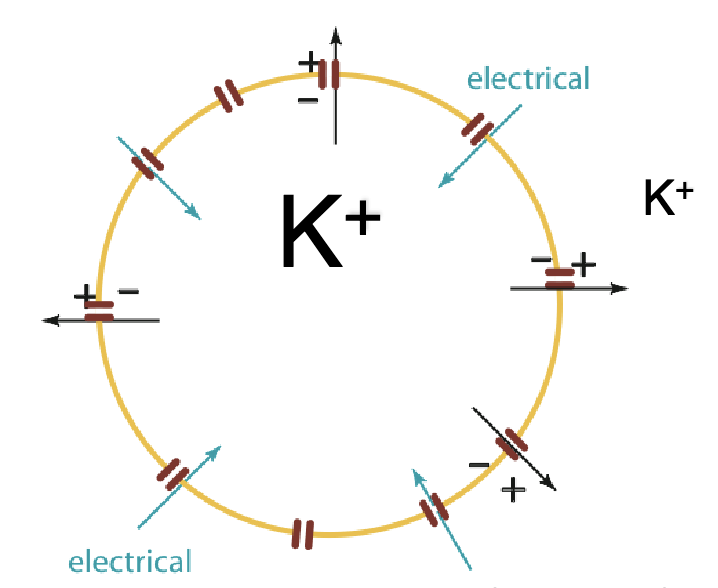
what equation describe the equilibrium condition of the generation of a membrane potential in a hypothetical cell permeable to K+?
Nernst equation
EK: membrane potential in mV
RT/F are constants
z: ion charge
[K+]o and [K+]in are the ion concentrations outside and inside the cell, respectively
![<p>Nernst equation</p><ul><li><p>EK: membrane potential in mV</p></li><li><p>RT/F are constants</p></li><li><p>z: ion charge</p></li><li><p>[K+]o and [K+]in are the ion concentrations outside and inside the cell, respectively</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/de53bdae-81e6-47e8-b854-3c4026e9f095.png)
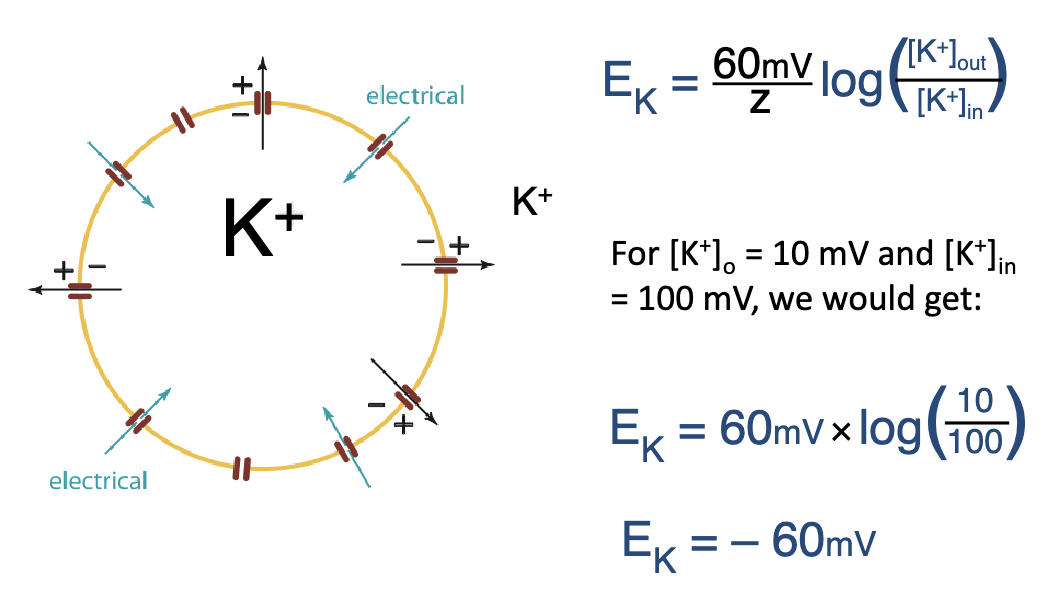
what does it mean that Ek = - 60 mV?
Ek is referring to the charge of the inside of the cell
Ek is referring to the charge of the inside/outside of the cell
inside
E-Cl = -60 mV means the inside of the cell is negatively charged
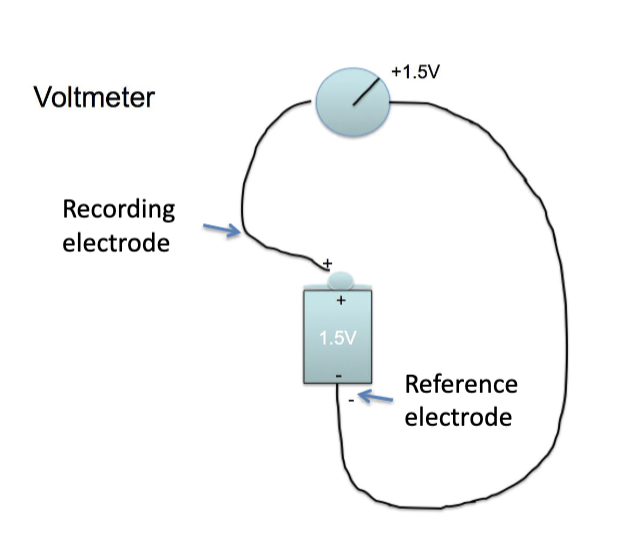
this is ebcause recording electrode used in voltmeter is always on inside of cell surface
what instrument is used to measure charge?
voltmeter
for a membrane whose EK = -80mV and is only permeable to K+ ions, would the concentration of K+ be higher intracellularly or extracellularly?
- the membrane is negative on the inside of the cell
- this implies that for a cell with K+ as the only permeable ion, K+ would have to have a higher intracellular concentration than extracellular

what is membrane potential?
electrical charge on membrane
measured in milivolts using a voltmeter
E (has polarity and magnitude)
Nernst equation only works for what situations ?
where there is only 1 permeable ion
In neurons, K+out is 4 mM and K+in is about 145 mM, then EK = 60 x log (4/145) = -93 mV
This is called the equilibrium potential for potassium or ____
EK+
For Na+, the outside concentration is about 142 mM and inside the cell about 12 mM, so if Na+ were the only permeable ion, ENa = 60 x log (142/12) = +65 mV
This is called the equilibrium potential for sodium or ___
ENa+
what 4 ions are important in neurons?
K+
Na+
Ca2+
Cl-
(listed E-ion is equilibrium potential for each ion if only that ion were permeable)
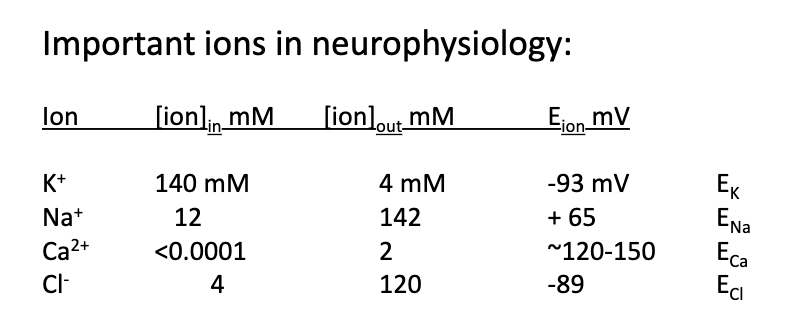
what happens in a hypothetical case with 2 permeable ions, Na+ and K+
equal concentrations
membrane is 2x more permeable to K+
2 K+s cross the membrane in the same time that one Na+ crosses
one of the K+s exchanges for the Na+, the other remains on the membrane- this leaves a net charge on the membrane of 1
law of electroneutrality doesn’t matter because we’re exchanging a positive for a positive ion
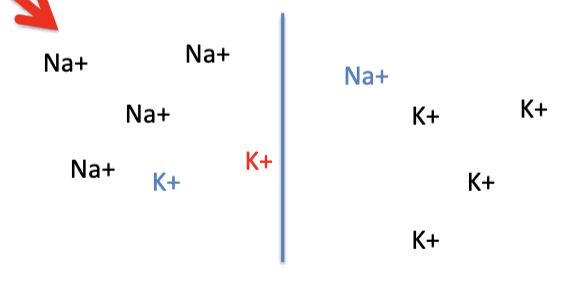
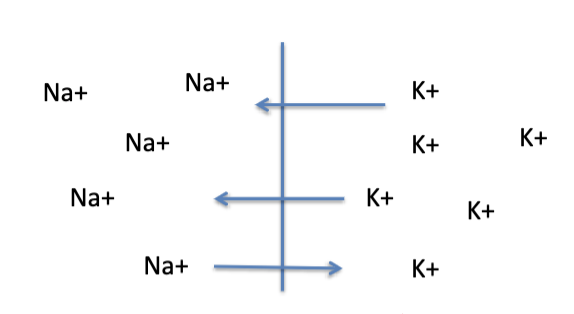
in neurons, both K+ and Na+ can diffuse through specific channels in the neural membrane.
E-Na: equilibrium potentials if only Na+ was permeable
E-K: equilibrium potentials if only K+ was permeable
Em: membrane potential if the 2 ions were equally permeable (NOT AN EQUILIBRIUM)

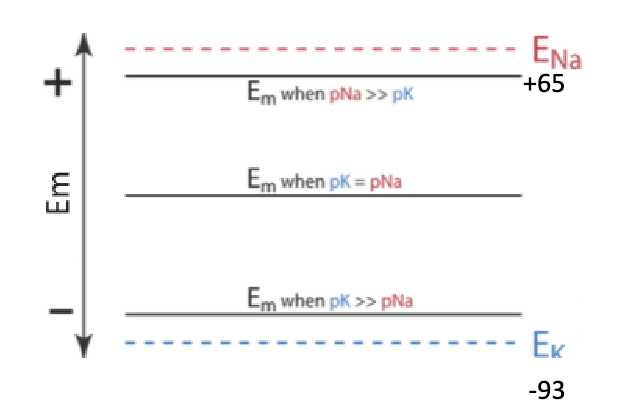
what is Em?
can be any value between E-Na and E-K depending upon relative permeabilities of the 2 ions
For example, if a membrane had a measured Em of -80 mV, we would know it was highly permeable to K+ (pK), but, since it was not at -93 mV, there was also some permeability to Na+ (pNa)
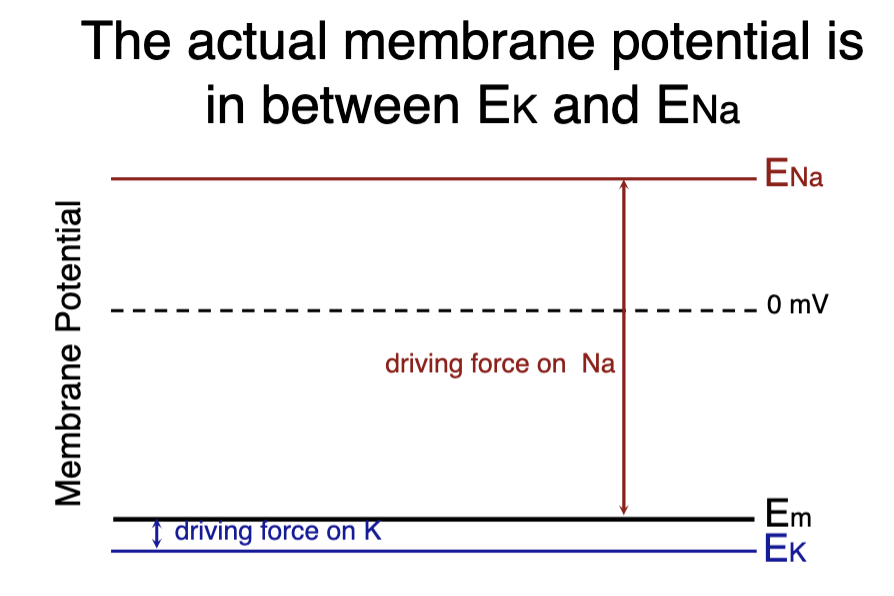
permeability of K+ is greater than that of Na+
what is resting potential of a neuron?
-80mV
typical Em for a resting neuron
If the permeability to any ion increases/decreases, the Em will move towards the equilbrium potential of that ion.
increases
a resting neuron is permeable to both Na and K - it has channels for both ions. the cell membrane contains what machinery to facilitate the movement of both these ions?
leak pores → small amounts of K and Na leak in/out diffusing down their concentration gradients
Na/K active transport system (pump) → returns diffusing ions to original sides to maintain concentration gradients of both ions
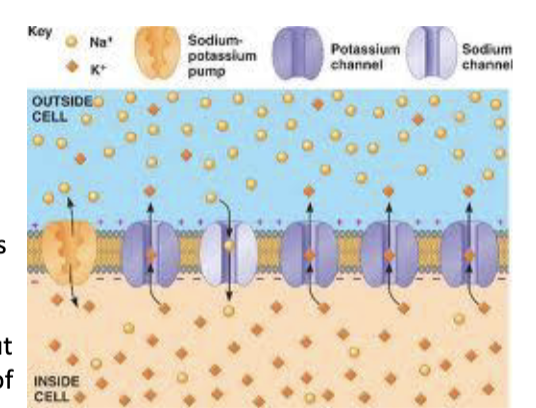
the resting potential, Em, of a typical neuron is in the range of -90 to -70 mV indicating a higher permeability for which ions?
K ions
thus, the membrane will have K ions on the outer membrane surface and negative ions on its inner surface
a pump uses ______ to move ions against their concentration gradients
cellular energy (therefore, resting state of a neuron is NOT at equilibrium)
The membrane potential, Em, is influenced only by ______ ions
permeable
how do ions move down electrochemical gradients?
passively
the influence of any ion on Em is proportional to that ion’s relative ________
permeability
what proportion of all available ions actually move to set up (or change) Em?
Only a very small proportion
t/f: In the short term, the ionic concentration gradients do not change significantly with changes in Em
true