oxidation reduction redox
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
What is oxidation state
A hypothetical charge of an atom assuming the bonding is completely ionic
What should the total sum of oxidation state be except for molecular ion
ZERO
But in molecular ion it should be the charge of the ion
What is the maximum and minimum oxidation state of an element
Maximum is its group numebr
Minimum is griup number - 8
What is the rules for o.s fo oxygen
-2 except peroxides where its -1
IN OF2 its +2
What is o.s of hydrogen
+1 but in metallic her=drides = -1
What is the o.s of group 1 ad 2
1 = +1
2= +2
What elements o.s vary
Transition elements and p block vary
In simple binary compounds the more electronegative has
The more electronegative event has negative oxidation state
D block elements o.s is
ITS SHOWN
CopperII= cu2+
What is dangerus about Fe(II) and Fe(III)
It can cause cell damage and cancer in th brain
That’s why measuring the oxidation state of fe in tumour - determines severity of cancer
All molecules ending in ——- contain —— block elements and some oxygen atoms
All molecules ending in TE contain d or p block element atoms and som oxygen atoms
How do you explain how a reaction is redox in an exam
Say what has been oxidised and show oxidation states
Say what has been reduced and show oxidation’s states
redox reactions occur when there is an oxidation and reduction reaction happening in the same reaction
What do oxidising agents do?
Accept electrons
They take the electron from the element being oxidised
How are oxididsmg agents used in our body?
HDROGEN PEROXIDE is formed - cause damage to cell and cause it to bust
Healthy cells can convert the altered DNA back to normal
Cancer+ ageing + heart disease slow down this rate
ANTIOXIDANTS (V.E or V.C0 remove oxidising agents from our body so slow down the alteration of DNA
What does cr2072- make
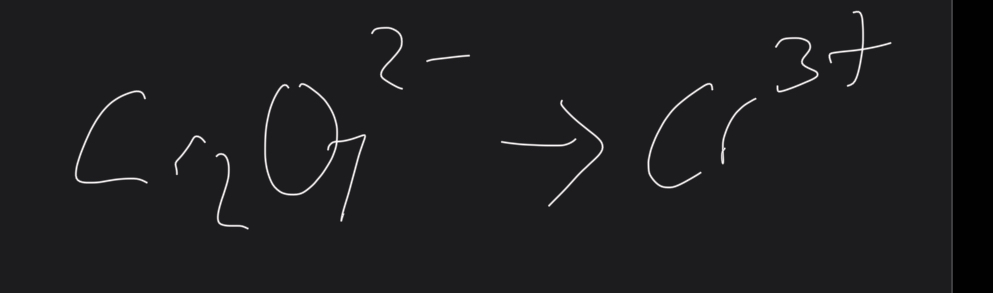
What is half equTIOSN
Involve electrons and only one species is either oxidised or reduced
Ionic equations do not involve electrons - 2 species are involved where one is oxidised one is reduced
2 half equations combined form an ionic equation
How do u do half equations
Multiply half equations to make same electrons in both equations
Add together and cancel
What do you have to make sure when combining half equations
That one is oxidastion and one is reduction equation
If there not - must rearrange it